Terrence P. O’Brien, MD, professor of ophthalmology, Charlotte Breyer Rodgers Distinguished Chair and director of the Refractive Surgery Service at Bascom Palmer Eye Institute of the Palm Beaches, agrees that there’s no gold standard test for dry eye. “Over the years we’ve had to rely on the patient’s history, plus symptoms and a composite of clinical tests to make a diagnosis—especially because the patient’s symptoms often don’t match the clinical signs,” he says. “The Schirmer test has less than 50 percent sensitivity; tear-film breakup time is more sensitive but lacks specificity. Corneal staining is primarily only helpful late in the course of the disease; and questionnaires, although they’ve been validated, only have about 80 percent sensitivity and 72 percent specificity. Clinicians and patients are literally crying for better objective diagnostic tests for dry eye disease.”
The need for better dry-eye diagnostic tools has spurred many researchers and companies into action, and the result is an increasing number of every-more-sophisticated instruments—some already available in the United States, and others still in the pipeline. Eight of the most promising are profiled below.
The First Wave: TearLab
One of the first high-tech diagnostic tools to appear was the TearLab Osmolarity Test. Although the test is widely used, there appear to be limits to how much can be determined by testing this one factor.
|
“The severity of the condition also matters,” he continues. “Less severe eyes, which are often more challenging to diagnose, have considerable variability in osmolarity readings, so the test is less useful. The device seems to work pretty well for eyes that have more severe dryness; in those cases, it provides a baseline of severity and gives you a way to track the recovery or normalization of the osmolarity in response to therapy. However, those usually aren’t the patients we debate about in terms of a diagnosis. So when diagnosing patients with less severe disease, we’ve found the TearLab platform to be most useful in combination with other tests, rather than by itself.”
“There’s no question that tear osmolarity goes up in dry eye, and there is a threshold,” agrees Stephen C. Pflugfelder, MD, professor and director of the Ocular Surface Center at Baylor College of Medicine’s Cullen Eye Institute in Houston. “The problem is that the TearLab instrument is fairly variable, so a single reading could be falsely low or high. The reliability improves with multiple readings, but that gets to be costly. In my opinion, a single measurement in the office is valuable primarily if it’s abnormally high. If the test is normal but you think the patient has dry eye, you’re probably going to go ahead and treat the patient regardless of the TearLab result.”
Dr. Rapuano says his clinic has used the TearLab machine for about six months. “Osmolarity is a reasonable surrogate for dry eye,” he notes. “We decided to try the TearLab because it was the most mature of the newer tests and seemed to have the most potential. Generally, the results confirm the impression we get from the slit-lamp exam. It is useful to have a somewhat objective number to look at; it gives us an idea of where patients are at this point in time.”
Dr. Rapuano notes that a key part of getting an accurate TearLab measurement is taking the measurement before anything else has been done to the eye. “You can’t put drops in, manipulate the eye or do an exam before using TearLab,” he explains. “It has to be done first. That means you can’t examine a patient and say, ‘Oh, you have dry eye, now I’m going to do a TearLab on you.’ In that situation, we usually tell the patient that we’ll do the TearLab test the next time he comes in, before anyone has touched his eye. When new patients come in, we have a little dry-eye evaluation form we ask them to fill out, to hopefully alert us to the problem before anything else happens.
“I think the TearLab is a nice addition to our armamentarium,” he concludes. “I don’t think it’s the end-all and be-all, but it’s helpful, especially when it gives a result I’m not expecting. If I think the patient has really bad dry eye but the tear osmolarity is low, then I look back and consider other possibilities I might not have been thinking about. If the osmolarity reading is unexpectedly high, I know that dry eye may be more of an issue than I initially thought. That only happens on occasion, but that’s when I find it most helpful.”
The RPS InflammaDry
|
Dr. O’Brien says he’s excited about the potential of using inflammation as a biomarker to aid in the diagnosis of dry eye. “A number of inflammatory markers have been identified in tears,” he notes. “Lactoferrin, lysozyme, cytokines and other tear-film markers offer great potential, and having the ability to detect inflammation can be useful both for diagnosis and tracking response to therapy. So we’re excited about having a device that can reliably and accurately detect levels of MMP-9 in the tear film.
“Increased MMP-9 can contribute to a number of problems, including disruption of the corneal epithelial barrier, decreased surface regularity and increased cell turnover,” he explains. “It really is a marker for dry eye; studies have confirmed that patients who have ocular surface disease with dry eye have elevated levels of MMP-9 in the tears. In fact, I think it’s a more sensitive diagnostic marker than clinical signs, and it correlates well with our exam findings. The sensitivity is 85 to 90 percent, with a specificity around 95 percent. That’s quite beneficial.
“Our data suggests that people without dry eye or other ocular surface disease have between 3 and 40 µg/ml of MMP-9 in their tears; above 40 µg/ml is elevated and abnormal,” he continues. “The readings correlate well with the different levels of dry-eye severity, based on the DEWS severity criteria. I think this is a good test, especially since it demonstrates a good correlation in milder cases, which can be problematic to detect with the osmolarity test.”
Is MMP-9 the best choice of marker to measure? “There are a variety of proteins altered in dry eye, but MMP-9 is one that’s consistently been shown to be elevated with dry eye and normal in people without dry eye,” Dr. O’Brien notes. “Clearly there are other conditions in which MMP-9 can be elevated, but those are obvious from the clinical presentation. Even though it’s only one matrix metalloprotease, it’s a common one that’s been well-studied and shown to be abnormal in these patients.”
Another advantage of measuring MMP-9 is that it appears to indicate a likelihood that certain treatments will be effective. “When MMP-9 is elevated above 40 µg/ml, patients will respond better to anti-inflammatory therapies, both corticosteroids and immunomodulatory agents like cyclosporine, tacrolimus and others,” says Dr. O’Brien. “Knowing the osmolarity, in contrast, is less helpful in terms of directing your therapy or predicting patient response to specific treatments.”
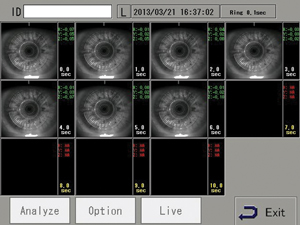
Monitoring Tear Film Instability
“One key to diagnosing tear dysfunction or dry eye is instability of the tear film,” notes Dr. Pflugfelder. “Instability is pretty much found in all tear dysfunction problems—including meibomian gland dysfunction, conjunctivochalasis, aqueous tear dysfunction and Sjögren’s syndrome—whether there’s a low tear volume or not. One new device that can help measure that factor is the Tear Stability Analysis System, or TSAS, from Tomey.
| ||||||
“In the study we conducted, we used this technology with dry eye of different clinical severity levels, from one to four,” he says. “We found a clear correlation: The more clinically severe the dry eye, the more rapidly the irregularity score went up. It provided valuable information, even early in the disease. It’s a test I would use on every patient, except that we can’t get reimbursed for it. Right now there’s no billing code. It’s approved, but not reimbursable.”
Meibography Plus
|
“We bought the Oculus Keratograph 5 because of the meibography, but we use it in a couple of ways,” explains Kelly Nichols, OD, MPH, PhD, FAAO— FERV Professor at the University of Houston College of Optometry and director of The Ocular Surface Institute at the university. “In terms of meibography, when taking images the instrument provides a little more guidance than previous models, such as showing you where to center the lid in the box. Once you’ve taken the picture, it automatically enhances the contrast of the image so you can get a clearer view of the glands. The company is also working on a grading algorithm for what’s normal and abnormal; I’m hopeful that in the future we’ll be able to compare a patient to an age-matched sample, or to previous images of the same patient.
“The Keratograph 5M also has tests that were not part of the previous model, as well as tests that have been enhanced, which the company refers to as the dry-eye suite,” she continues. “That includes a tool for measuring tear meniscus height. Once you have a photograph of the eye, you use a little onscreen widget tool that looks like a Roman capital letter I. You put the top bar of the capital I at the top of the tear meniscus, and the bottom bar at the lid margin; the program then gives you an exact measurement. This provides a level of accuracy that you couldn’t get looking through a slit lamp, even with a reticule. Also, because you’re measuring an image, you don’t have to worry about the patient moving. You can take measurements all the way across the lid margin, if desired.
“Actually, we use this more in clinical research than in clinical practice,” she notes. “I believe that in practice a global assessment of whether the patient has tear prism or not is almost as good as having a quantified measure. But it might be valuable for comparing how contact lenses affect the tear film, or if the eyes have significant differences in epiphora.”
| ||||||
Dr. Nichols says the instrument also quantifies conjunctival redness. “To do this, you take a color photo,” she says. “When you click the button, it creates a grayed-out circle in the center of your view which you overlay on top of the iris and pupil to mask them out of the picture. Then you press go, and the instrument gives you a measure of limbal and conjunctival redness. This is mostly helpful as an objective way to monitor improvement and show the patient that you’re making progress but that’s sometimes a valuable service.”
Dr. Nichols notes two other, more qualitative measurements that can be made with the instrument’s dry-eye suite at the push of a button. “The device allows you to view the lipid layer of the tear film, giving you an interferometry pattern,” she says. “A lot of colored fringes indicate a thicker lipid layer. There’s no measurement map associated with that, but if you use it a lot, I believe you could learn to gauge whether the lipid layer seems normal or not. This is similar to what the LipiView does, although the LipiView goes one step further; it measures the color of the output and gives you a number—an average lipid layer thickness.
“The device also allows you to see the speed at which the tear film moves upward after a blink,” she adds. “You focus two white dots on the tear plane. As the patient blinks, you can see how fast the tear movement is occurring on the ocular surface, without any need to use a dye. A fast speed is normal; if it’s very sluggish, or you see a lot of speckly stuff, that would be another indicator of an irregular tear film or lipid layer.”
Dr. Nichols notes one other feature of the Keratograph that she appreciates: the camera. “For the money, it has a fantastic camera,” she says. “It wouldn’t be ideal for some purposes, such as taking pictures of corneal degenerations or small things on the cornea that require high magnification and a slit beam; but for a general photograph of the ocular surface—cornea, conjunctiva, lids, lashes, even fluorescein and tear breakup time, it provides a fantastic picture. Onscreen, you can digital zoom, fix the lighting, turn the gain up or down and make real-time adjustments. It takes better fluorescein photographs than any other method I’ve seen, without having to do any optimization. In contrast, most cameras attached to a slit lamp aren’t optimized for viewing through a cobalt filter, for example.
“My opinion of all the extra tests in the Oculus dry-eye suite is that they do add some didactic value,” she says. “Clinically, I don’t know if I would use all of them; I probably would still do my regular tear breakup time test, and I’d look through the slit lamp at the elements of the tear film moving and tear meniscus height, etc. In any case, aside from the dry-eye tests and meibographer, this is a topographer first and foremost. But given all the functions it performs, I think you get a lot for your money. And like the LipiView, it’s a step toward a functional, more objective way to evaluate the tear film and ocular surface in dry eye and other common diseases.”
Dr. O’Brien’s Ocular Surface Center clinic also has an Oculus Keratograph 5M. “This technology allows us to evaluate the condition of the meibomian glands; many people are now saying this may be useful in diagnosis,” he says. “The preliminary results are encouraging, in terms of being able to detect MGD and tearfilm breakup time with more quantitative accuracy. The chief problem with diagnosing dry eye has been the semi-quantitative nature and variability with all of the tests, so the more quantifiable, reproducible and accurate the test, the better it will be. Oculus and several others are showing great promise as a means to provide that.”
Another new meibography tool is a portable, handheld, pen-shaped device developed in Japan and manufactured by Topcon. The non-contact device incorporates an infrared LED light source and a sensitive video camera that can be connected to a monitor or computer; it provides a panoramic view of all of the meibomian glands along the upper or lower eyelid, without causing the patient any discomfort. The device is available for import but is currently not approved by the Food and Drug Administration. (For more on this, see “Breaking New Ground in Meibography” in the July 2013 issue of Review.)
Analyzing the Lipid Layer
|
“The LipiView test is designed to measure the thickness of the lipid layer, but we have not found that to be very helpful,” he continues. “It doesn’t seem to correlate too well with people’s slit lamp exams or symptoms, or with what we think of as the severity of blepharitis. I’m not sure whether that’s a technological issue, or if the thickness of the lipid layer really isn’t a determinant of lipid layer function. It doesn’t really help us determine whether the patient is a good candidate for LipiFlow; instead, I determine that based on the slit lamp exam and the symptoms. Nor does it correlate with whether the patient is successful with LipiFlow.
“The most interesting part of the LipiView test, in fact, is the little video of the patient blinking that you record as part of the test,” he says. “The device gives you a printout that shows you who the partial blinkers are. This has nothing to do with the lipid layer thickness that the instrument is measuring, but for many patients, a partial blink is a big part of their problem. Unfortunately, there’s no easy way to eliminate partial blinking, but at least we can tell the patient, ‘This is why the 20 medications and treatments you’ve tried for this issue have not worked. You’re not completely blinking, so the lower half of your cornea is getting dried out as the day goes on.’ ”
Dr. Nichols also has used the LipiView device. “In practice, we’ve found patients with seemingly normal lipid layer thickness on the LipiView test; but you look at their secretions and their meibography and they’re not normal,” she says. “Maybe they just rubbed the eye and expressed some lipid. There’s a lot we don’t know yet, but it’s clear that you can’t use any one of these tests alone.”
Dr. O’Brien says the Ocular Surface Center clinic at Bascom Palmer Eye Institute has a LipiView device, but has only recently been gaining experience with screening dry-eye populations. “It produces very intriguing high-tech images, but the interpretation is still difficult,” he says. “I’m not sure we fully understand yet exactly what the patterns mean or how they correlate to clinical disease. However, it’s been useful for catching partial blinkers, and it’s been useful as a patient education tool, letting us show patients why they’re uncomfortable or having problems.”
The TearScan MicroAssay
Another new tool designed to allow quantification of key markers in the tear film is the TearScan MicroAssay System from Advanced Tear Diagnostics; it measures markers relating to both dry eye and allergy. “There’s a lot of art to treating dry eyes, and the science is lagging behind,” notes Young Choi, MD, medical director of InVision Ophthalmology in Homewood, Ala., who has used the TearScan MicroAssay System for almost a year. “However, I think the diagnostic machines are starting to catch up. This instrument, for example, is able to quantify both lactoferrin and IgE in the tears. It’s extremely sensitive and specific. It’s reproducible and accurate, and we know that lactoferrin level is indicative of dry eye. So it tells you a lot about your patient’s condition.”
Dr. Choi says the fact that the MicroAssay System also measures an allergy-related marker (IgE) is very helpful. “One of the big advantages of this system is that the IgE reading tells me if the problem is more irritation from allergy than dryness,” he says. “There’s a lot of overlap between aqueous-deficiency dryness, evaporative dryness and allergy dryness.
“This kind of consistent, reproducible data will help tease out some of this overlap and help us fine-tune our treatment regimen,” he notes. “We won’t have to do a shotgun approach and put every patient on the same thing. Maybe we can say, ‘Yours is really more of an aqueous production problem; let us help you make more tears.’ Or, ‘Yours has an allergy component to it, so we’ll treat for both allergy and dry eye.’ Now we’re addressing more of the actual problem.
“Of course, this is all new; no one has been routinely measuring lactoferrin in the tear film,” he says. “We don’t fully understand what the role of lactoferrin is. For that reason we’re gathering data to get a better idea of the clinical consequences of different lactoferrin levels. Does this indicate more aqueous deficiency dry eye, or evaporative dry eye?”
Dr Choi says he believes this information will become even more useful as time goes on, and may have a role beyond just diagnosing and treating dry eyes. “For example, this could be an important tool for screening LASIK patients, because lactoferrin may make a difference in outcomes,” he says. “An ARVO poster a few years ago reported an association between lactoferrin level and whether LASIK eyes came out over- or undercorrected. Since it gives us a precise number, we may be able to associate specific levels of lactoferrin with specific amounts of over- or undercorrection.”
Dr. Choi says he believes the TearScan MicroAssay System is ready for prime time. “It’s only a few thousand dollars, which is a fraction of the cost of some other machines out there,” he observes. “The data is consistent and reproducible, and there’s evidence that lactoferrin levels are associated with dry eyes. You have something you can hang your hat on. I see this as a big step forward in terms of putting more science into diagnosing and treating dry eyes.”
Diagnosing Dry Eye with OCT
“Several of the currently available optical coherence tomography devices can help the clinician determine whether the tear volume is reduced, by noninvasively measuring tear meniscus dimensions,” says Dr. Pflugfelder. “That tells us whether the patient has an aqueous deficiency or not, which can point the diagnosis in a different direction. We use the Optovue OCT for this; Zeiss makes the Cirrus OCT, and there may be other OCTs with this capability. In fact, our Optovue has software specifically designed to measure the tear meniscus dimensions.
|
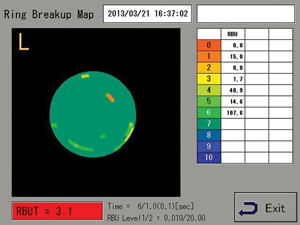
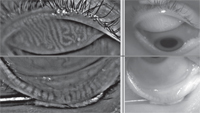
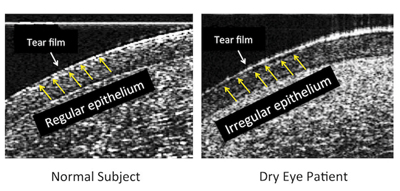
A number of Dr. O’Brien’s colleagues at Bascom Palmer have been working on the possibility of diagnosing dry eye using real-time high-resolution OCT. “Our group has developed a prototype custom-built ultra-high-resolution spectral domain OCT device, or UHR-OCT, to study the ocular surface of dry-eye patients in a noninvasive fashion down to resolutions of 2 to 3 µm,” explains Victor L. Perez, MD, associate professor of ophthalmology, microbiology and immunology at the Bascom Palmer Eye Institute. “Utilizing UHR-OCT we’ve microscopically mapped the ocular surface; we’ve found that dry-eye patients have specific surface irregularities on their corneal epitheliums that can be measured and quantified using a diagnostic index that we’ve named epithelial irregularity factor, or EIF. (See example, right.) Those epithelial irregularities are most likely the microscopic structural injurious effects that dry-eye syndrome imposes on the ocular surface. Those microscopic structural changes seem to be readily detected by the unprecedented high resolving power of recently developed imaging devices such as our UHR-OCT.
“Our preliminary data has demonstrated that our novel dry-eye diagnostic index, the EIF, provides a non-invasive qualitative and quantitative means to diagnose dry-eye syndrome that correlates accurately with patients’ signs and symptoms,” he continues. “Moreover, our preliminary data have shown that EIF could be a tool for objectively and subjectively monitoring dry-eye response to treatment, and could be used to develop and test new therapies.”
“These results are really exciting,” he adds. “They show that EIF could address many of the limitations of the currently available diagnostic techniques. Spectral domain OCTs with such high resolution have already moved from research laboratories to clinics, and commercial models will soon become available to help improve the standard of care of our patients.”
Harvesting Cells for Testing
Although removing cells from the surface of the eye and sending them to a laboratory for testing is a more complex approach than some of the new point-of-service tests, it offers the advantage of allowing a far more detailed analysis—and the testing of the cells may soon become an in-office capability. Ironically, one reason this has been little used in the clinic is the difficulty of sampling cells from the ocular surface. Some companies are trying to address that.
|
Currently, the device simply harvests cells from the conjunctival surface, but the company is working to develop it into a point-of-service test for dry-eye markers. “For example, you might sample the cells and discover that the ocular surface is missing mucous cells,” he says. “That could explain the dry eye. And of course, we could also test for inflammatory biomarkers that are exploited by the cells when dry eye is present. Polymerase chain reaction testing is another possibility we’re exploring.
“The device is simple to use,” he continues. “You open the sterile, single-use pack and ask the patient to look down or up, depending on the area being sampled. You place the tip against the conjunctiva and gently press the plunger for a couple of seconds. Then you remove the device from the surface of the eye and eject the sample into the container that will be used for shipping to the laboratory.
“The conjunctiva is a mucous tissue that is constantly renewing itself, like skin,” he notes. “The EyePrim samples the most superficial layer of cells, cells that are about to be ejected into the tear film. The cells simply adhere to the surface; there’s no scraping involved. The device is very safe, and the patient doesn’t feel anything. There’s no visible change on the surface of the eye, and the sampled area heals within a matter of hours.”
To test the efficacy of the device Mr. Roy says the company has conducted several clinical studies, one of which was done with a team from Birmingham, England. “They found that this procedure is about two times faster than the old procedure,” he says. “It’s also more efficient; they retrieved enough material for testing in 100 percent of the cases. On average, the device collected two to five times more cells than the older procedure, without the pain. That’s especially important in a clinical trial, because if you sample patients three times over the course of the trial and 20 percent of your sampling fails to produce sufficient material for testing, you could have a 50-percent data loss. The ease of use was also clear; nurses are now using the device to take the samples instead of the doctors.”
The EyePrim is currently being used in several clinical trials to measure biomarkers and evaluate the efficacy of the different treatments that are progressing toward marketing. The device, approved but not yet reimbursable, is distributed in the United States by Ocular Surface Diagnosis Innovation in Tampa. OSDI also offers biomarker-based diagnostic services, so samples can be shipped to them for testing.
Dr. Pflugfelder has used the EyePrim. “This kind of test would be very useful for diagnosing dry eye,” he continues. “It’s well-known that patients with aqueous tear deficiency show a decrease, and sometimes an absence, of goblet cells. Furthermore, the severity of irritation, light sensitivity and corneal/epithelial disease has been found to correlate with the number of goblet cells. So this would be an important parameter to measure. Also, Restasis increases the number of goblet cells; that may be one of its major mechanisms of action. So this might be a way to identify patients who would be good candidates for that treatment. In any case, the EyePrim is a great device.” REVIEW
Dr. O’Brien has been a non-salaried ad hoc consultant/advisor for Rapid Pathogen Screening, TearLab, Advanced Tear Diagnostics and Tear Science. Dr. Choi is a consultant for Advanced Tear Diagnostics. Drs. Shousha and Perez have submitted a United States Provisional Patent Application for the HR-OCT technology. Dr. Nichols has no financial interest in Oculus or TearScience or their instruments. Drs. Pflugfelder and Rapuano have no financial ties to any company or product mentioned.