Like everything else in medicine, however, that protocol has slowly been evolving—especially the ways in which antibiotics are used. Initially, many surgeons had their patients use antibiotic drops for a period of time before and after surgery. Now the use of antibiotics before surgery has come into question, and alternatives to the use of postoperative drops are proliferating. In particular, intracameral injection of antibiotics, with or without steroids, is becoming more widely accepted.
Here, four surgeons with experience using intracameral antibiotics share their thoughts on the pros and cons of different approaches.
Catching the Wave
“There’s definitely a change in the dynamic of what we’re doing to manage complications post-cataract surgery,” says Francis Mah, MD, who specializes in cornea, external disease and refractive surgery at Scripps Health System in San Diego. “More and more prospective studies are showing the efficacy of intracameral antibiotics. Outside of the United States, I think the majority of surgeons believe that intracameral injections are superior to drops, based on the medical literature. In the meantime, there’s plenty of interest in this kind of protocol here. The latest ASCRS survey of cataract surgeons showed that close to 80 percent were interested in it, or would definitely do it if there were an FDA-approved agent. However, only 20 to 30 percent of U.S. cataract surgeons are actually doing routine intracameral right now.”
|
William F. Wiley, MD, director of the Cleveland Eye Clinic, agrees that this type of approach is gaining traction. “Almost all of my colleagues across the country are either considering using an injection at the end of cataract surgery or are already doing it,” he says. “They realize that the cost of traditional medications has gotten out of hand and an alternative approach is reasonable. At a recent meeting I heard four or five lectures on the subject of minimizing postoperative drops, so awareness of the issue is spreading. There’s definitely a need to try to decrease the expense and inconvenience of traditional medication.”
Samuel Masket, MD, a clinical professor of ophthalmology at the Jules Stein Eye Institute, UCLA School of Medicine in Los Angeles, and a past president of the American Society of Cataract and Refractive Surgery, notes that the idea of intraocular antibiotics isn’t really new. “It took the European Society of Cataract and Refractive Surgery study1 to capsulize the significant positive difference made when intraocular antibiotics are used,” he says. “But if we go back more than 20 years, two cataract surgeons known for their contributions to the field and significant patient volumes—Jim Gills, MD, and Howard Gimble, MD—were routinely either infusing or injecting antibiotics at the end of surgery. And they were not alone. When I surveyed the ASCRS membership in 1996 and 1997 there was already significant use of intracameral agents. Out of 1,270 surgeons who replied to the survey, 18 percent were using intraocular vancomycin.
“Despite this level of interest, it never really got the attention that it should have,” he adds. “One of the problems was proving the efficacy of such a protocol; it wasn’t until the ESCRS study that the idea really began to gain traction.”
One Surgeon’s Protocol
Dr. Masket describes the steps he takes when using intracameral antibiotics. “Since roughly 2007 I’ve used 50 µl of Vigamox, which is moxifloxacin, directly out of the bottle, injected into the anterior chamber as the last thing we do at surgery,” he explains. “I want to stress that it’s µl, not ml; it’s half of one tenth of a milliliter, a very small amount. We only do this once we’ve checked that all incisions are hermetically sealed, whether with sutures or ReSure ocular sealant. We’ve tested the incisions with point pressure, we’ve done an operative Seidel test and we’ve set the intraocular pressure at physiological levels, as measured with a tonometer, so we know we have a sealed, non-leaking system. Once that’s accomplished, the last thing I do is inject the Vigamox directly into the anterior chamber. I’ve done this for at least eight years and have not seen one case of infection. I’ve used it in each and every eye, irrespective of the procedure I’m doing, although of course the great majority of surgeries are cataract surgery.” He acknowledges that some surgeons use a slightly different protocol. “Some people inject 0.1 µl rather than .05 µl,” he says. “Also, some surgeons dilute it, which I don’t believe is necessary.”
Dr. Masket points out that the only agent that can be injected in this way happens to be Alcon’s Vigamox. “It’s not intended for intracameral use,” he notes. “This is strictly an off-label use. But Vigamox happens to have a pH of 6.8, which is similar to aqueous; and its osmolality, or tonicity, is about the same as aqueous. Plus, it has no preservative. For those three reasons—tonicity, pH and absence of preservative—off-the-shelf Vigamox is compatible with the intraocular environment and is tolerated intracamerally without dilution.”
But Is It Safe?
Given that many surgeons have achieved minimal endophthalmitis rates using this type of protocol, one might wonder why more surgeons aren’t adopting this approach. Part of the reason is undoubtedly the absence of an injectable that’s FDA-approved for this purpose, along with the respectable results associated with more traditional protocols. But there is also some concern about some poor outcomes that have been reported, often relating to factors such as compounding problems.
|
“One way around these concerns is to avoid having to compound the injected drugs,” he continues. “In Europe there is a French company, Thea, which manufactures an approved single-use injectable antibiotic for delivery inside the eye, sidestepping this problem. It comes premixed, requiring a single dilution. I believe the product is approved in 20 or 30 countries outside the United States, and I believe they have approached, or plan to approach, the FDA in this country. Other companies around the world are undoubtedly also working on drugs for this purpose that will not need to be compounded, and many surgeons in the United States are waiting for an FDA-approved, single-use intracameral antibiotic.”
Studies that sidestep the compounding concern have generally been more positive. Dr. Masket notes that he was part of a three-site study that also included Stephen Lane, MD, and Robert Osher, MD. “We did a masked investigation that was published in 2008,2 in which at the end of surgery we’d either inject Vigamox or BSS,” he explains. “Then we looked at corneal thickness as a measure of corneal toxicity, OCT as a measure of macular toxicity and endothelial cell counts. We also looked at IOP and a few other parameters. We found no difference between BSS and out-of-the-bottle Vigamox with respect to toxicity or safety. Of course, this was not a study of efficacy. One would have to do thousands of cases to determine whether the protocol was efficacious.”
Dr. Masket notes that the idea of using vancomycin has generated serious pushback. “The antibiotic that Drs. Gills and Gimble recommended was vancomycin,” he says. “There was one study that compared two groups of patients, one with vancomycin infused, the other not, that found a higher incidence of cystoid macular edema in the vancomycin group. But it was a poorly done study that was refuted by numerous surgeons. Nevertheless, it’s still occasionally quoted as a reason not to use vancomycin. In contrast, you have a study like the ESCRS study, which was well-designed and executed. Some people criticize it on the grounds that they stopped the study too soon for the numbers to be as significant as they should be, but they did that because the result was so obvious to them. They had a fivefold increase in infection when they did not use intracameral agents. They didn’t feel they could continue the study for moral reasons.
“I believe it’s inappropriate to discount the findings of the ESCRS study,” he adds. “Besides, several other studies from well-known researchers, including the one I conducted with Drs. Lane and Osher, have shown that a protocol like this is safe and efficacious, especially when using moxifloxacin. But until the day comes when the studies have convinced the FDA that intracameral antibiotics are safe and efficacious, we’re not going to have readily available single-dose units. Unfortunately, as far as the FDA is concerned, there is currently no irrefutable proof that intraocular antibiotics reduce the rate of infection.”
Surgeons and others have also expressed concern about whether this type of protocol might increase antibiotic resistance. “The academic medical centers and the Centers for Disease Control, as well as the American Academy of Ophthalmology, have expressed concern about this,” explains Dr. Masket. “This was considered especially important because vancomycin was the last treatment option for certain enterococcal infections outside the eye. But because vancomycin has low toxicity, and because there had never been a gram-positive infection in the eye that was found to be resistant to it, a lot of people have used it anyway, over a long period of time.”
Dr. Masket doesn’t believe that resistance is likely to be generated by using vancomycin inside the eye. “The problem is that if you’re running it through bottles that eventually end up in the trash, a small amount of antibiotic could get washed out into the environment,” he says. “However, the use of vancomycin and fluoroquinolones in industry and agriculture is massive—especially in agriculture. In contrast, it’s been estimated that only 0.02 percent of the antibiotics being used are used in the eye, so it’s very unlikely that we make a meaningful contribution to antibiotic resistance.”
Alternative Delivery Methods
In addition to intracameral injection, other methods have been used (and are being developed) that can provide antibiotic coverage following cataract surgery.
• Infusion. Some surgeons have simply added the antibiotic to the fluid going into the eye, either in addition to an injection at the end of surgery or as a stand-alone approach to endophthalmitis prevention. “The problem with infusion is that it’s hard to know exactly how much medicine is getting in and whether the concentration is adequate,” says Dr. Masket.
|
“Some doctors manage this by both infusing during the case and adding a bolus of antibiotic at the end of the case,” he continues. “The concerns about the bolus at the end come down to errors in dilution. This is the result of what we call ‘dealing with a kitchen pharmacy.’ That’s why the use of Vigamox has been so appealing to me. I know it’s off-label, but I don’t have to rely upon a circulating nurse or somebody else in the OR to make a decision about how to dilute the agent. In contrast, if we’re going to inject something by bolus, then it does have to be diluted, and if a mistake is made, which unfortunately does occur from time to time, you may induce significant toxicity and end up with TASS. That can be sight-threatening. Those incidents are very rare, but they’re enough to cause many centers and hospitals to believe it’s not safe to dilute agents and place them inside the eye. That has certainly impacted the acceptance of this type of protocol. And unfortunately, even though I found the ESCRS study very convincing in terms of efficacy, that study used a second-generation cephalosporin called cefuroxime. There are questions regarding whether cefuroxime is the best or most appropriate agent. Perhaps more important, it requires preparation.”
• Subconjunctival injection. Dr. Mah notes that subconjunctival injection of antibiotics has fallen out of favor. “Studies have suggested that the way the drug gets into the eye following a subconjunctival injection is similar to eye drops,” he says. “It ends up getting in through the cornea. It simply sticks around for a longer period of time because there is a depot. How beneficial this approach is is debatable in the medical literature. I think an equal number of studies have found it to be beneficial vs. showing no clinical benefit. Anyway, in this age of topical anesthesia, patients don’t want to have a subconjunctival hemorrhage, and they don’t want the pain associated with the injection. So I think the subconjunctival approach is less and less popular nowadays.”
Despite these concerns, Dr. Gills used a subconjunctival injection for the steroid part of his protocol so his patients wouldn’t have to use steroid drops following surgery. “Within the past five years, we’ve used kenalog injections rather than topical steroids,” he explains. “We inject 1.2 cc behind the limbus, subconjunctivally at 2 o’clock, sub-Tenon’s. We don’t put it into the anterior chamber because it blurs vision and the drug dissipates very quickly. With a sub-Tenon’s injection, it lasts about six weeks. However, you have to make sure the injection is more than 8 mm away from the limbus because if you get any type of steroid deposit within 8 mm of the limbus, there appears to be a rise in pressure in a certain percentage of patients.”
Dr. Gills admits that doing a subconjunctival injection of steroids carefully and accurately adds about 10 percent more time to the cataract surgery. “We do it because it’s good for the patients, and our patients like it,” he says. “A lot of our patients come in and say, ‘I want that surgery where I don’t have to go and buy all of those drops.’ I think it’s a practice-builder.”
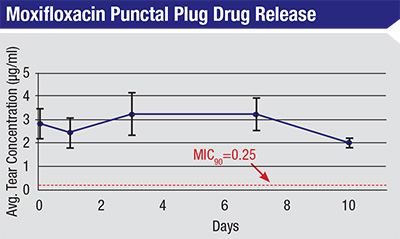
• Devices that slowly release antibiotic. Dr. Mah notes that several companies are working on developing unique direct-delivery systems or devices. “Ocular Therapeutix is already conducting a clinical trial of a punctal plug delivery system that elutes moxifloxacin,” he notes. “PolyActiva in Melbourne, Australia, has developed a bioerodable implant that releases levofloxacin over a 30-day period. Other companies are also hoping to bring delivery systems to market. One big challenge, of course, is getting any such system approved by the U.S. Food and Drug Administration, especially when its use is related to cataract surgery. This could involve conducting studies with hundreds of thousands of patients. Plus, they face a second hurdle: Getting the drug paid for by Medicare and insurers.”
• Optimized topical drops. Dr. Wiley notes that Imprimis now also makes a combination drop that can be used after LASIK or cataract surgery. “It’s the same theory, just not as dramatic a change from the more traditional protocol,” he says. “For post-LASIK the traditional regimen is an individual steroid drop and an individual antibiotic drop. The most common medications for this purpose are a prednisolone steroid and a fourth-generation fluoroquinolone. We noted that there was no eye drop combination of a fourth-generation fluoroquinolone and prednisolone, so because we’ve had success using TriMoxi for cataract surgery, we asked Imprimis if they could make an eye drop that would combine steroid and antibiotic.
“This made sense for three reasons,” he continues. “First, it’s much more convenient for the patient, reducing the number of drops by 50 percent. Instead of using two bottles, patients are just using one. Second, it lowers the expense to the patient dramatically. Right now our LASIK patients are spending $150 to $200 for the two individual drops. With the combination product, the cost would drop to $50 or so. Third, it’s much more convenient for the surgery center. The drops can be delivered straight to the center, so we don’t have to call in different drops for different patients at different pharmacies. When surgery day is coming up, we just say, OK, we have 20 patients, these are the names. We send the list to our pharmacy and they create the drops for those patients and send them to us. They’re waiting for the patients after surgery. And of course, the same idea could be used following cataract surgery; those two medications are very common in cataract surgery.”
|
A Combination Injectable
Although no product is FDA-approved for this purpose, some companies are offering options that may simplify preparation by having multiple drugs combined in one injectable product. For example, when performing cataract surgery, Dr. Wiley uses Imprimis’s TriMoxi, which combines triamcinolone and moxifloxacin.
“Unlike many other options intended to serve this purpose, TriMoxi is compounded, but it’s not made in a commercial pharmacy,” he notes. “You can have a local compounding pharmacy make something similar; however, you should make sure the local pharmacy is reputable and licensed, as there are well-recognized concerns regarding compounding. Also, Imprimis has some proprietary techniques for formulating the triamcinolone so it can go through a smaller needle; the drug is dissolved to a smaller molecular size than traditional triamcinolone. We place the TriMoxi in the vitreous at the time of cataract surgery with the goal of decreasing or eliminating the need for eye drops. Using TriMoxi in this way is off-label—but currently there are no compounded or commercially available antibiotics that are considered on-label for addressing endophthalmitis after cataract surgery.
“Before we switched to this approach we had our patients use three different medications for one month after surgery,” he continues. “Right now, we still have our patients use one drop postoperatively, once a day for a week, so we haven’t eliminated drops altogether. The drop we’re using now is called Maxitrol; it’s a combination antibiotic and steroid. Mostly, it’s there just to cover the patients in case they need something postoperatively. They won’t have to rush to the pharmacy to get a drop if they have an issue in the perioperative period. It also makes patients feel good to have a drop, and I’m more comfortable knowing they have that resource. It’s never been clinically proven that using antibiotic drops after cataract surgery prevents endophthalmitis, but it makes me a little uncomfortable to go from traditional drop protocols to no drops at all. After we get a comfort level and refine the current regimen, we’ll probably stop even using that drop. In fact, many practices nationwide are going completely dropless using the same kind of technique.”
Dr. Wiley says they’ve done about 500 cases using this protocol and have seen no endophthalmitis. “Nationwide and internationally, I know of a number of colleagues who are using the same or a similar technique, and they’ve been happy with their endophthalmitis prophylaxis,” he says. “So it seems to be working very well. Meanwhile, our patients like the simplicity and the cost-savings. Some of our patients were paying upwards of $600 to $800 for branded medications around the time of cataract surgery. We found that some patients were holding off on having cataract surgery because they couldn’t afford the medications. In contrast, Maxitrol is available on a generic basis, easy to obtain, very inexpensive (under $20) and simple to use—once a day for a week. Now patients feel more comfortable proceeding with the surgery.”
Dr. Wiley points out that many surgeons currently inject an antibiotic at the end of cataract surgery, but not a steroid. “Over the past decade or so many surgeons have been injecting Vigamox, a commercially available antibiotic,” he says. “They dilute it themselves at their surgery center or hospital and inject it at the time of surgery. And it’s fairly common for doctors to use vancomycin. In fact, the use of antibiotics inside the eye around the time of surgery is not new; the newer thing is combining it with a steroid and placing it in the vitreous.”
Dr. Masket says he’s not ready to try the combination-drug injectables from Imprimis. “The current delivery concept is to put the agent into the vitreous through the zonules,” he says. “You put a cannula into the anterior chamber, poke it through the zonules so that it goes into the vitreous; then you’ve got the mixture sitting in the vitreous. I am not enamored of the concept of a transzonular delivery system; I think it needs further investigation. There are obvious potential downsides to it, although I’m very interested in studying it in the lab.
|
“I agree fully with the concept of going dropless when we can,” he says. “Imprimis is moving in that direction, but this requires a huge difference in the way we deliver medication, and it violates, to me, some of our surgical principles—specifically, to not disturb the zonules. My partner and I have spoken about participating in an investigation using it as a pars plana injection rather than a transzonular injection. However, we tried that in one case and ended up with a very milky vitreous and a complaining patient. So I’m not yet ready to accept that mode of delivery. I applaud Imprimis for what it’s doing, and those doctors who are investigating it, but I think we need a very carefully designed study to look at the risks and benefits.”
Dr. Wiley says he understands why surgeons would be concerned about these aspects of the TriMoxi protocol. “We are putting triamcinolone into the vitreous, and it’s a whitish medication,” he says. “It is visible floating in the vitreous, so for the first 24 to 72 hours patients do notice a fair number of floaters,” he says. “However, we manage this by talking with the patient pre-operatively and setting appropriate expectations. We were worried about the decreased ‘wow effect’ we might see, but in reality it hasn’t been a concern.
“As far as potential issues with trans-zonular application, there are two approaches to placing the medication—transzonular and transcleral using a pars plana injection,” he explains. “The latter is similar to what retinal specialists do when giving intravitreal injections for macular degeneration, which has become one of the most common procedures performed worldwide. It’s very safe and effective. That’s the approach I’ve chosen to inject the TriMoxi, because it’s easier for me to perform than the alternative and there’s no risk of disrupting the zonules. In any case, there are many different techniques regarding how much steroid people put in at the time of surgery and where they put it. Some people put it all in the vitreous; some put it in the vitreous or the anterior chamber; some put it in the vitreous, anterior chamber and/or sub-Tenon’s.”
Unanswered Questions
Not surprisingly, some important questions remain:
• Which antibiotic is best for intracameral injection? Dr. Mah notes that it’s not yet clear which antibiotic is the best choice. “Most experience with this around the world has been with cefuroxime,” he says. “Its effectiveness has been demonstrated in numerous studies. There have been some papers looking at moxifloxacin, though, as potentially having some superior characteristics. A United States study from Kaiser Permanente in northern California actually found moxifloxacin to be superior to cefuroxime or vancomycin. I believe there’s also a Japanese retrospective study that found moxifloxacin to be at least as good as cefuroxime.
“One factor may have influenced the seeming superiority of moxifloxacin in the Kaiser study,” he continues. “They did not use cefuroxime intracamerally if patients had a broken capsule; but they did use moxifloxacin if patients had a broken capsule. That could have influenced the results, because the literature supports the idea that patients with broken capsules are at increased risk of infection. Using moxifloxacin in higher-risk patients may have helped to reduce the statistical incidence of endophthalmitis, compared to not using any intracameral agent.
“In any case, the majority of prospective studies have involved cefuroxime,” he says. “Which antibiotic is most effective is still debatable. But the biggest debate, initially, is going to be the concept of intracameral antibiotics. Many surgeons are not yet convinced that this approach can be trusted as their primary prophylaxis for infection. We’re just beginning to try it in our own surgical center, but after reviewing all the literature, I believe the data, and the data indicates that this is a better method of preventing infections than the traditional protocol using drops. However, the issue of possible TASS must be incorporated to calculate overall positive vs. negative effects of the technique.”
• Do patients need postop antibiotic drops after an intracameral injection of antibiotic? “That’s an issue that has not been resolved,” says Dr. Masket. “It depends in part on when the eye is at risk. We do our surgery, we put antibiotic in, we think the eye is sealed, we think we’re safe. However, the eye is still susceptible to contamination postoperatively until the epithelium seals over the incisions. That can take two or three days, so during that period that patient is still potentially subject to infection from contamination—particularly if the wound is unstable. For that reason, I think we still have a potential need for eye drops in the early postoperative period. I typically keep patients on drops for five to seven days, even though I use intracameral antibiotics; I want to protect the patient from post-op contamination, not just intraoperative contamination. Unfortunately, the half-life of moxifloxacin is very short, unless it’s in repository form. If it’s gone within 12 to 18 hours, then it’s no longer protecting the patient.”
Dr. Masket adds that a sealant like ReSure might solve this problem without postoperative drops. “A sealant may keep the incision closed for three to five days,” he notes. “That could be a very excellent substitute for postop drops.”
• Do patients need antibiotic drops before surgery? “I think most surgeons agree that using povidone iodine at the time of surgery should be standard,” says Dr. Mah. “However, there isn’t any data to support the use of topical antibiotics ahead of surgery as a way to prevent postop endophthalmitis—although some people look at the decrease in colony counts and see that as surrogate evidence that preop topical drops may help prevent postsurgical infections. Having said this, there isn’t any evidence that it does not help, either.”
The Coming Thing?
“There are many things we need to keep in mind in order to decrease infections postoperatively,” says Dr. Mah. “But I do think surgeons will be moving toward intracameral injection of antibiotics, whether it’s sooner or later. It will happen sooner if we can get an FDA-approved medication for this purpose.”
Dr. Wiley agrees. “In five years, I see the use of drops being drastically reduced or eliminated, unless studies find some problem with the alternatives,” he says.
“It will be great when we can supply the patient with a single dose of medication at the time of surgery that manages prophylaxis against infection, as well as other issues such as inflammation,” says Dr. Masket. “Everybody is moving toward drug-delivery systems that eliminate the need for drops. We all know that drops are really inefficient; they come with different rates of absorption, poor compliance, toxicities, and now, unfortunately, huge expense. So all of us would love to eliminate them.
“People out there are developing drug-delivery systems that will be safe and efficacious,” he adds. “Given all the nanotechnology in the pipeline, I’m sure in the reasonable future we’ll see agents that combat inflammation and infection that are placed inside the eye during surgery. At that point, eye drops will only be used under certain circumstances. I can’t say how far off that is, but I know it’s coming soon.” REVIEW
Dr. Mah is a consultant to Alcon, Allergan, B+L/Valeant, Ocular Therapeutix and Polyactiva. Dr. Wiley does paid research for Imprimis and Ocular Therapeutix. Dr. Masket has no relevant financial ties.
1. Beselga D, Campos A, Castro M, Fernandes C, Carvalheira F, Campos S, Mendes S, Neves A, Campos J, Violante L, Sousa JC. Postcataract surgery endophthalmitis after introduction of the ESCRS protocol: a 5-year study. Eur J Ophthalmol 2014;24:4:516-9. doi: 10.5301/ejo.5000417. Epub 2013 Dec 16.
2. Lane SS1, Osher RH, Masket S, Belani S. Evaluation of the safety of prophylactic intracameral moxifloxacin in cataract surgery. J Cataract Refract Surg 2008;34:9:1451-9. doi: 10.1016/j.jcrs.2008.05.034.