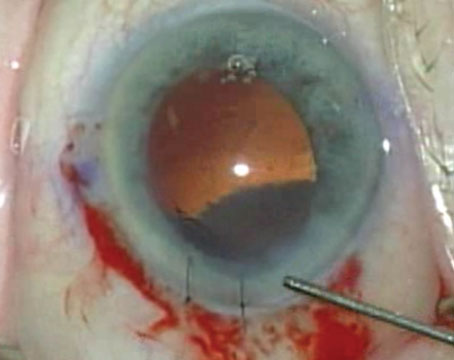
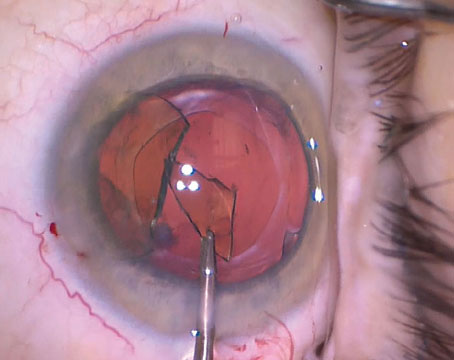
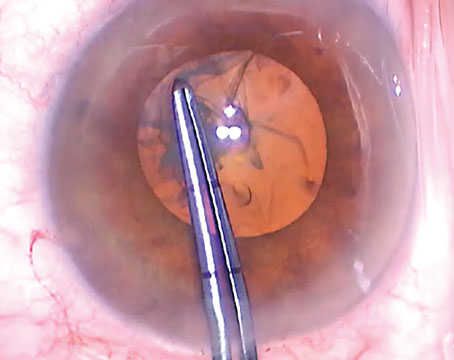
Part of the reality of cataract surgery is that a small percentage of implanted monofocal intraocular lenses and presbyopia-correcting intraocular lenses eventually require an IOL exchange. Those exchanges are necessitated by numerous problems, including pathology, surgical error and patient dissatisfaction with refractive outcomes or visual phenomena.
A recent study conducted at Duke University Eye Center explored the causes of lens exchange and the success of different methods used to perform the exchange. The study involved a retrospective review of 91 eyes of 83 patients who underwent IOL exchange between January 2015 and April 2019; lenses explanted included 66 monofocal IOLs and 25 presbyopia-correcting IOLs. (Fifty-six percent of patients with monofocal IOLs and 40 percent of patients with presbyopia-correcting IOLs had had other prior ocular surgeries.)
The study (presented at the 2020 meeting of the American Society of Cataract and Refractive Surgery) found:
- Sixty-three percent of the exchanges were for dislocation; 8.8 percent were done to address uveitis-glaucoma-hyphema (UGH) syndrome.
- Eighty percent of presbyopia-correcting lens exchanges were done to address lens-induced visual disturbance.
- Monofocal IOLs were most often replaced with an anterior chamber IOL; presbyopia-correcting lenses were most often replaced with a ciliary sulcus posterior chamber IOL.
- Patients with both monofocal and presbyopia-correcting lenses had improved UCVA and BCVA following IOL exchange.
- Prior ocular surgery may be a risk factor for IOL exchange.
Here, surgeons share their insights and pearls for deciding when an exchange is appropriate and how to make sure it leads to the best possible outcome.
Deciding How to Proceed
“When you’re doing an IOL exchange you have to have a plan A, plan B and sometimes a plan C,” notes Kevin M. Miller, MD, chief of the Cataract and Refractive Surgery Division of the David Geffen School of Medicine at UCLA. “Very infrequently, you’ll have a situation where everything is perfect except the power of the lens. Then you can stick with plan A and swap out the off-lens for an appropriately powered lens. But that only happens five percent of the time in my practice. Most cases are much more complicated.
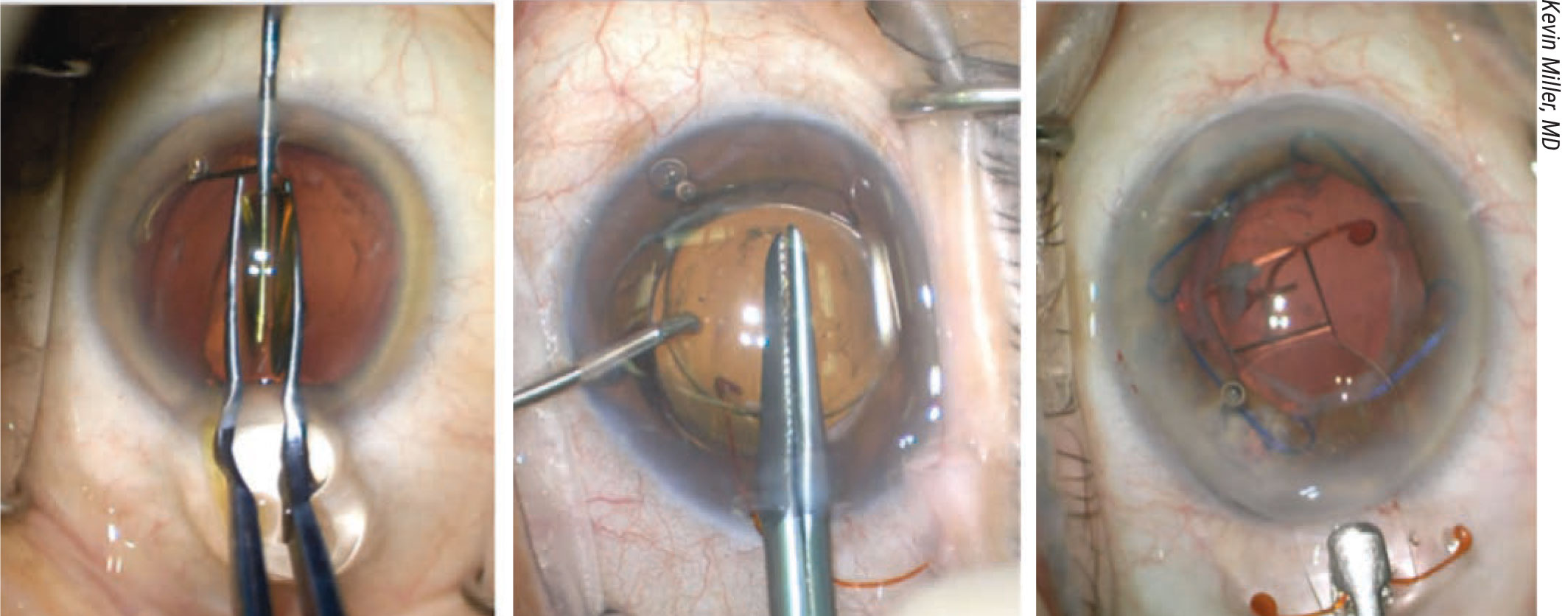 |
| There are three ways to explant a problematic lens: fold it to reduce its size; cut it partially or completely; or remove it in one piece. |
“Once you’ve established the need for a lens exchange, there are multiple scenarios to consider,” he continues. “There are basically three places inside the eye where you can put a lens: the capsular bag, the ciliary sulcus and the anterior chamber. A lens exchange can involve removing a lens from any one of these three spaces and then placing a new lens into any one of them. Capsular bag to capsular bag is common for addressing lens-power errors. If the capsule is torn, and the lens is decentering because it’s in the torn bag, you might take it out of the bag and put it in the ciliary sulcus. If the entire capsular bag is dropping onto the macula, you might take the lens out and put the new one into the anterior chamber or suture it to the sclera. In some cases, the lens you’re removing may be outside the bag as well.
“Of course, you can subdivide these three locations further,” he continues. “The lens can be inside the capsular bag or partly captured in the bag, as when the optic is captured in the capsulorhexis or even the posterior capsule, and the haptics are in the sulcus. A lens can be passively placed in the sulcus between the iris and anterior lens capsule, or it can be actively fixated in the sulcus—sutured to the iris or sclera, for example. Or, the haptics can be fixated in the sclera using Amar Agarwal’s glued-IOL technique or by putting them through the sclera, as in the Yamane technique.
“Needless to say,” he adds, “if you have to remove a lens that’s already in one of these other locations, the process will be very different from an in-the-bag exchange.”
Does research suggest that one technique is more successful than another? “One of the purposes of our study was to compare the different techniques used for secondary IOLs and IOL exchanges, to see if one technique stood out as better or worse,” explains Melissa B. Daluvoy, MD, an assistant professor at the Duke Eye Center in Raleigh, North Carolina, and fellowship director of the Cornea/Anterior Segment Division, who coauthored the study described earlier. “Our study found that the tried-and-true methods we’ve used for many years—such as anterior chamber IOLs—were just as good as some of the new scleral-fixated techniques. There was no statistically significant difference in outcomes, in terms of visual outcomes or complications. The conclusion we’ve drawn is that whatever you’re good at doing is probably the technique you should use for secondary or replacement IOLs.”
Reposition or Exchange?
Kenneth J. Rosenthal, MD, FACS, an associate professor of ophthalmology at the John A Moran Eye Center, University of Utah, and surgeon director at Fifth Avenue Eye Care and Surgery / Rosenthal Eye Surgery in New York, notes that the decision about whether to exchange or reposition a wayward lens depends on a number of factors. “If I’m dealing with a younger patient who’s had the lens in their eye for more than eight or 10 years, I almost always take out the old lens,” he says. “I do that for a couple of reasons. Number one is that today’s lenses don’t last forever. When we first started doing lens implants, most were made of PMMA, which lasts for a very long time. But most lenses today are made out of acrylic—or less commonly, silicone. Those lenses are more perishable, meaning that over time, various things happen to them that affect the clarity of the optic, and even more commonly, the integrity of the haptics.
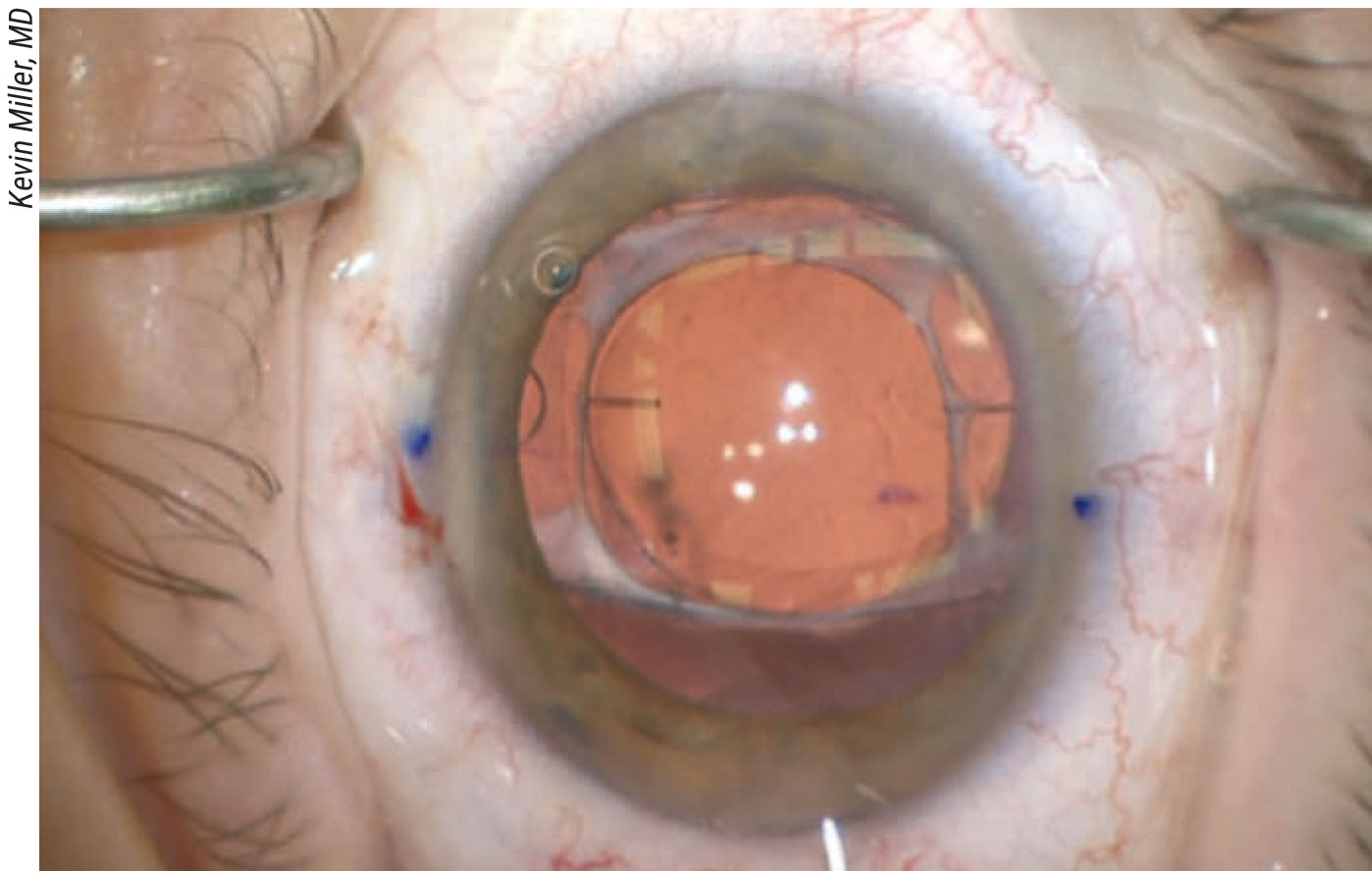 |
| This patient had a STARR silicone toric plate haptic lens—the first toric lens ever made available. (Note the axis marks and one of the positioning holes, partially visible to the left.) Asymmetric capsular fibrosis decentered the lens, causing the patient to have double vision when sufficiently dilated. Rather than risk a recurrence following lens repositioning, the surgeon broke up the fibrosis, explanted the lens, placed a monofocal lens in the bag and performed corneal relaxing incisions to address the astigmatism. |
“For example,” he continues, “glistenings and opacification of the lens occur over time. In addition, many of the three-piece lenses have haptics made of polypropylene that become brittle over time. As a result, these lenses can have limited longevity in some patients. Furthermore, when we do surgery on these patients we manipulate the lens, and we may actually cause micro-damage. If you’re repositioning the lens rather than replacing it, that damage can ultimately cause the lens to fail.
“For that reason, if I have a 50-year-old patient who may live another 30 to 50 years, I’d rather put a new lens in,” he says. “It’s kind of like putting a new tire on your car rather than plugging the old tire. Besides, in an otherwise healthy eye, the amount of surgical manipulation that’s involved in removing a lens and exchanging it for a new one isn’t substantially greater than just repositioning the existing lens, given modern techniques employing viscoelastics, thorough pars plana vitrectomy and small-incision surgery.”
Dr. Rosenthal notes, however, that there are exceptions to this. “I’d consider repositioning rather than exchanging the IOL in a patient who has a lot of retinal pathology,” he says. “Another exception is patients who have decreased corneal endothelial cell counts, where you want to get in and out stealthily with the least amount of surgical manipulation. A third exception would be a very elderly patient who can’t tolerate a longer procedure, and where the expected lifespan may be shorter.”
One situation in which the decision about whether to reposition or explant a lens can be tricky is when dealing with a subluxed lens. “Usually that’s a decision I make on the fly,” says Doug Grayson, MD, a surgeon at Omni Ophthalmic Management Consultants in Iselin, New Jersey. “I prepare to do either option. However, if it’s a one-piece lens, that’s coming out.”
Dr. Daluvoy agrees. “If the problematic lens is a single-piece lens, I think you should take it out,” she says. “Most of the scleral-fixated or iris-fixated techniques only work with a three-piece IOL.”
“If you have a subluxed three-piece lens, then you have options,” Dr. Grayson continues. “Those lenses are always still in the bag, and the bag is kind of a mess around the lens. You may be able to localize the haptic that’s visible on the three-piece, throw a suture under the haptic, pass it through the iris and do a McCannel-style fixation. But sometimes that’s not easy to do, especially if the lens is almost falling.
“Keeping the lens in there and doing a Yamane fixation can be a little tricky too,” he says. “You have to get all of the old capsule off of the lens, and then do your Yamane externalization of the haptics. Unfortunately, the existing lens won’t be a CT Lucia lens, which is best-suited to the Yamane technique because it can withstand a lot of manipulation without the haptic cracking off—although the Tecnis lenses are pretty durable for using the Yamane technique as well.
“The other issue is time in the OR,” he adds. “It takes a fair amount of time to clean off the existing lens and suture it in place. Other options, like doing a vitrectomy, putting in a well-fitting AC-IOL and doing an iridotomy take far less time. And, the patient will see great the next day.”
Placing a Lens Outside the Bag
Other options besides placing the lens in the bag or fixating it may be worth considering.
“Everybody is currently Yamane-happy,” notes Dr. Grayson, “but an AC-IOL is fine. No study has shown that there’s a long-term difference in vision between a well-fit AC-IOL and any of the fixated IOLs. In fact, with an AC-IOL you won’t have problems with lens tilt, and in my experience you’ll have a lower risk of CME. If the anterior chamber is deep and the lens fits right and is well-placed, you won’t have a risk of corneal-endothelial compromise. Sometimes you simply don’t have enough space in the orbit to do a fixated lens, so I’m prepared to do Yamane or an AC-IOL, and I explain this to the patient as well.
“If I’m dealing with an older patient and the cornea looks relatively healthy, I think an AC-IOL is a reasonable approach,” says Dr. Daluvoy. “I tend to avoid an AC-IOL in younger patients because it can cause some endothelial cell loss as time goes on. For the younger patients I typically prefer to put the lens scleral fixated behind the iris, if that’s an option.”
“Some surgeons like to implant a piggyback lens,” Dr. Grayson adds. “There are situations in which this may be a good alternative, such as when a corneal refractive fix is off-limits and there’s no way to easily remove the existing lens from the bag. I tried implanting piggyback lenses for a while. It’s great until you get some kind of posterior iris surface chafing and secondary pigment-dispersion glaucoma.”
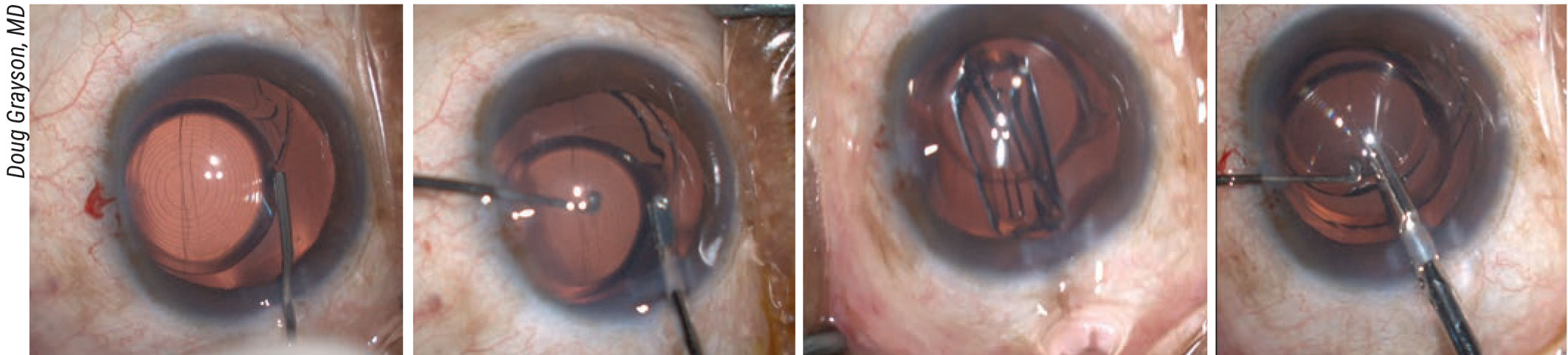 |
| One popular technique used in lens exchange involves placing the new lens in the bag before removing the old lens from the eye, as a way to protect the bag from being damaged during the removal process. A) The existing IOL is mobilized using viscoelastic. B) The existing lens is maneuvered out of the capsular bag. (Note the minimal fibrotic adhesions associated with the Symfony IOL.) C) The replacement IOL is placed in the capsular bag below the old lens. D) The old lens is cut in preparation for removal. |
Dr. Grayson says one of the situations in which a piggyback lens might make sense is when a high myope or astigmatic patient is out of range for any available toric or multifocal lens, but still wants multifocal or astigmatic correction (and has a lot of room between the iris and the IOL). “Basically, you put in the lowest power toric or multifocal lens you have,” he explains. “Then you see where the patient is visually, and compensate with a piggyback monofocal. Some surgeons using this approach will put both lenses in at the same time, but I feel more comfortable waiting to see what the exact refraction is with the first lens, six weeks later. Then I put in the appropriate piggyback IOL.”
Dr. Rosenthal says he doesn’t implant piggyback lenses much anymore. “I’ve done some in the past, but today the optic range of the lenses that are available off the shelf is pretty large. I do use a piggyback IOL to correct IOL power errors, on rare occasions. If you to decide to use a piggyback lens, the lens in front should always be a three-piece lens, never a one-piece lens. And always use a lens made of a different material from the existing lens, such as silicone on top of acrylic. In particular, avoid ending up with two acrylic lenses, because they tend to form intralenticular opacities.”
Getting the Lens Out
“There are basically three different ways to get a lens out of the eye,” explains Dr. Miller. “You can fold it; you can cut it; or you can take it out whole. My preference is to refold the lens, which is easiest. This won’t work with every lens; PMMA and silicone lenses can’t be folded. However, you can refold acrylic lenses because they’re soft and malleable inside the eye. Collamer lenses can be folded, although it’s sometimes a little tough to do. Silicone lenses are too slippery and thick in the center, so they resist being folded.
“The size of the incision you need to remove a lens changes depending on how you remove it,” he continues. “When you refold a lens to remove it, you’ll need a larger incision than you needed to inject the same lens. However, the incision can be a little smaller if you bisect the lens. I’d say the typical incision is about 3.5 mm when I’m folding a lens; when I cut the lens, the average is about 3 mm.
“Once a lens is removed, I place a suture in the corneal incision in about half of my cases—the ones that don’t seal well,” Dr. Miller says. “If I put a suture in, I usually take it out about a week later, and then do a final refraction two weeks later. If it’s a large incision, such as the incision you’d make to remove an intact lens that can’t be folded, I place multiple sutures. Then I wait two weeks after the final suture is out before doing a refraction for glasses.
“Sometimes,” he adds, “when I try to fold a lens the iris starts looking like it might come out through the incision. If that happens, I stop. Instead, I’ll just cut the lens and remove it.”
“If I decide to cut the lens, I prefer to cut about two-thirds to seven-eighths of the way across and then pull the lens out—essentially in one piece, but pulling out half at a time,” says Dr. Rosenthal. “Folding most lenses is easy; you use an old-fashioned cross-action lens folder. A cyclodialysis spatula goes under the lens; the folder goes over the lens. You fold the lens down over the spatula, then turn the lens 90 degrees and explant it. Removing a folded lens does require a slightly larger incision than you may need if you cut the lens. But folding the lens avoids the potential problem of the sharp edges of a cut lens shredding tissue on the way out.”
Dr. Grayson sometimes makes a cut about one-third of the way through the optic and then rotates the lens out using wound-assist folding. “If you pull it out slowly enough you won’t rip the lens, but you do need that cut to pull it out easily,” he explains. “You can do this under topical anesthesia; it’s pretty straightforward and safe.”
What about correcting a refractive error on the cornea? Kevin M. Miller, MD, chief of the Cataract and Refractive Surgery Division of the David Geffen School of Medicine at UCLA, notes that when deciding whether an exchange is necessary, lasering the cornea is sometimes an option. “If it’s just a refractive error, it might be possible to perform laser vision correction instead,” he says. “With patients in the cataract age range, it’s not going to be LASIK in my practice because of the high likelihood of dry eye; it will probably be PRK. However, if a patient has a hyperopic error of +2 or +3, I won’t get a very good result doing a hyperopic corneal treatment. In that case, I’ll swap out the lens. If it’s a myopic error such as -2 or -3 D, I might consider doing laser vision correction. “In reality,” he adds, “exchanging lenses because of a power error is one of the least-common procedures I do, however. It’s much more common for me to exchange because of an UGH syndrome, decentration or dislocation into the back of the eye.” Dr. Miller points out a practical issue that comes into play regarding corneal laser refractive-power correction. “Unless you’ve talked to the patient ahead of time about the possibility of doing laser vision correction,” he explains, “it’s going to be a hard sell after the surgery is done and the patient finds out you put a lens with the ‘wrong’ power lens in. Now when you tell the patient, ‘We’re going to leave the lens in and fix the problem by operating on a different part of your eye,’ the patient will say, ‘Absolutely not! Put the right lens in!’ If you didn’t have that conversation ahead of time, the patient will believe you did something wrong. Thinking you made a mistake, the patient will want you to fix the mistake. At that point the patient will have zero interest in laser vision correction.” —CL |
In the Presence of Capsulotomy
“If the patient has already had a YAG capsulotomy, then the likelihood of the vitreous coming forward is a lot higher,” notes Dr. Daluvoy. “If that’s the case, you have to be ready with multiple options, because things may not go exactly the way you’ve planned. Initially, I might plan on a sulcus-fixated IOL, if the anterior rhexis is still good. But if for some reason you lose the bag or there’s another problem, then you have to have a plan B, whether that be implanting an AC-IOL or a scleral-fixated IOL. I wouldn’t necessarily start with a pars plana vitrectomy in that situation, but I’d be ready either to go pars plana and do a vitrectomy or an anterior vitrectomy, if I encounter vitreous.”
“If the patient has already had a capsulotomy, the bag is no longer stable,” notes Dr. Grayson. “That means we can’t put a new lens into the bag, which eliminates our ability to do a good multifocal lens exchange. We can replace an existing multifocal with a monofocal, but in that situation I’d put a three-piece monofocal in the sulcus with optic capture through the capsulorhexis.
“In these cases I believe a vitrectomy is necessary,” he continues. “It’s almost impossible to exchange a lens without having some vitreous prolapse after a YAG capsulotomy, because the YAG doesn’t just affect the capsule; there’s no longer any anterior hyaloid protecting the vitreous. In that scenario you’re just asking for trouble if you’re not planning a vitrectomy at the time of the exchange. I like to put in pars plana trocars and do a core pars plana vitrectomy. Then I leave the trocars in—they’re self-sealing. After that I do the lens exchange. If there’s any vitreous left floating around at the end, I’ll just get rid of it.”
“A small- to moderate-size posterior capsulotomy, like those most surgeons would make, is not a contraindication to lens exchange,” says Dr. Rosenthal. “However, doing a lens exchange in those circumstances does require some specialized surgical techniques and more expertise than when dealing with an intact capsule. In many of these cases you’ll want to do a limited, pars-plana-approach vitrectomy, to remove the vitreous immediately behind the capsular opening before you elevate the IOL out of the bag. Also, when you dissect the lens free, be very gentle. Be aware of the anterior-posterior force you’re applying.”
“At the very least,” Dr. Daluvoy adds, “if I’m trying to exchange a lens because of quality-of-vision issues—meaning I want to change the power, or the patient is unhappy with a multifocal IOL—the presence of a capsulotomy changes my conversation with the patient and plays a lot into my preop decision-making.”
One thing everyone agrees on: When a patient has complaints after the primary surgery has implanted a multifocal, surgeons should never reflexively do a YAG capsulotomy. “Sometimes after you put in a multifocal, the patient will complain of glare and haloes and difficulty seeing at night,” notes Dr. Grayson. “That’s probably attributable to the multifocal optics. Don’t just do a YAG in hopes it will solve the problem, because if you do, you’ll have a patient with a multifocal lens and non-adaptation issues who will now need a vitrectomy.”
Dr. Rosenthal agrees. “When surgeons have difficulty with a lens implant after the primary surgery, the last thing they should be doing is a YAG capsulotomy,” he says. “Always explore other options first, and only do a YAG when you’re sure the patient doesn’t need a lens exchange.”
Removing Different Lens Types
Surgeons agree that lens exchange can be a very different proposition depending on which type of lens you’re hoping to remove. “Some popular lenses are made of injection-molded acrylic, which sticks more tightly to the capsule,” explains Dr. Grayson. “In addition, the haptics have a bulge at the end. Because the lens is more adherent, it causes a more aggressive capsular fibrosis; then, once it’s scarred into position, the bulge at the end of the haptics prevents you from rotating the haptics out easily. As a result, if you’re exchanging one of these lenses five or six weeks out—depending on the amount of scarring—sometimes you have to decapitate the haptics and leave them in place. That’s OK if you’re implanting another lens and you can orient the new haptics 90 degrees away from the old ones. But if it’s a power miss, or an axis miss with a toric lens, you can’t always put the new lens where it should be.
“In contrast, some other lenses are tumbled acrylic that’s lathed down and polished rather than molded,” he continues. “In this case the acrylic is much less adhesive and the haptics don’t have that bulge at the end. As a result, it’s much easier to exchange them a couple of months after the surgery. That’s great, because occasionally you need more time for the refraction to stabilize, whether the patient has epithelial membrane dystrophy, dry eye or is post-LASIK or post-RK.”
Dr. Miller agrees that the geometry of the lens makes a difference in terms of how difficult the lens will be to remove. “Loop-haptic lenses are generally very easy to get out of the bag,” he notes. “You can usually just dial the lens and the haptics will come right out, even if you have significant fibrosis of the anterior capsule. However, some popular lenses are very difficult to remove. Some have a terminal bulb at the end of the haptic, and you can get a ring of fibrosis around the neck of the haptic just proximal to that bulb. It’s usually impossible to pull the haptic through the ring of fibrosis—and the haptic is difficult to see, because it’s hidden behind the iris.
“The hardest lens to get out is the Crystalens, because it generates fibrosis around the four polyamide haptics,” he adds. “You can get such dense fibrosis that it’s really difficult to get that lens out. You can’t dial it or slide it because of the lens design; it’s trapped on both sides.”
Planning the Exchange
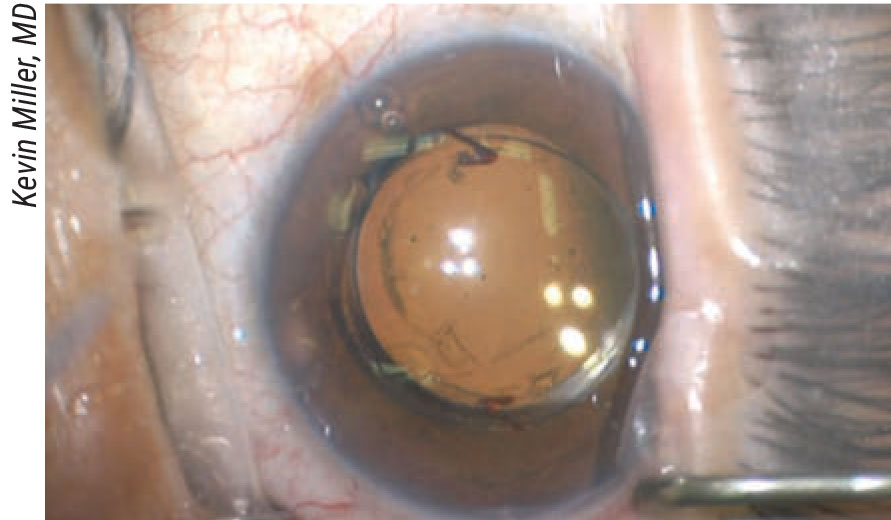 |
| Every case is unique. Here, a piggyback lens had been placed above an in-the-bag lens to try and resolve dysphotopsias. (It didn’t work.) Both lenses had to be removed, followed by a vitrectomy because the capsule had been opened. A new lens was placed in the sulcus, finally resolving the dysphotopsia problem. |
These preoperative strategies will help to ensure a good outcome:
- Don’t assume a lens in the bag can’t be explanted because it’s been in place for more than six months. “I hear this all the time, but it’s simply not true,” says Dr. Rosenthal. “I’ve exchanged lenses that have been in the bag for a decade. Yes, the more time has passed since the surgery, the more difficult it is to remove the lens; there will be more adhesions, and it will require a little more skill to disengage and dissect the lens free of the capsular bag. But there’s nothing sacrosanct about six months.”
- Always get an endothelial cell count as part of the preoperative workup. “The surgeon has to know the condition of the endothelium in order to make a decision about how to proceed with the surgery,” Dr. Rosenthal explains. “The surgical technique may need to be modified, and the condition of the endothelium should certainly influence your decision about whether to exchange a lens or reposition it. For example, if the patient has a very low endothelial cell count, repositioning an existing lens might give the endothelium a better chance of surviving.”
- When planning to scleral-fixate a lens, be meticulous about your measurements. “Be sure to place the lens the right distance back from the limbus so that it’s not too close to the iris,” says Dr. Daluvoy. “If it’s too close to the iris it may rub against it, causing UGH syndrome. Also, make sure your placement of the IOL is symmetrical so you don’t have lens tilt.”
- If the patient has glaucoma, you may be able to address that during the exchange surgery. “In some cases, I may do a goniotomy in conjunction with a lens exchange,” notes Dr. Grayson. “I’ve treated patients who’ve had secondary lenses, were already aphakic, or had a subluxed lens due to zonular compromise, when securing the existing lens wasn’t an option. If a patient like that has glaucoma, I’ll do a goniotomy when I put in the secondary AC lens.”
- If a patient has dysphotopsias with a monofocal in the bag, consider placing the new lens in the sulcus. “Putting the new lens in the bag might result in the same problem,” Dr. Miller points out. “Putting the replacement lens in the sulcus should help avoid the problem. Just be sure to adjust the lens power to compensate for the change in the effective lens position.”
During the Surgery
Surgeons offer these tips to make the exchange surgery go more smoothly:
- Do what you’re comfortable with. “If you have the skills and you’re comfortable with the tools, then learning and using the newer techniques is reasonable,” says Dr. Daluvoy. “But if you only do one or two exchanges a year, then do what’s safe and what you’re used to—place an AC-IOL or scleral-fixate the lens.”
- Consider sliding the new lens in before removing the old. Dr. Grayson says he frees up the old lens, lifts it into the sulcus and puts the new lens in the bag before removing the previous lens. “This way, the capsule is protected when you have to make a cut in the previous lens to explant it,” he explains.
“I’ve used that technique in the past when the posterior capsule was intact,” notes Dr. Daluvoy. “It depends on how stable everything looks. If there’s already a YAG capsulotomy, I’d take the first lens out and make sure my support is good before having two lenses in there.”
- When removing a lens, don’t be stingy about the size of the wound. “Today, surgeons often try to be valiant about minimizing the size of the wound,” notes Dr. Rosenthal. “In my experience, any downside of making a larger incision, such as induction of astigmatism or the need for a suture, is mitigated by the greater safety of having an adequate wound size to remove the lens. I typically use a 2.75-mm keratome, and then open the sides of the wound a little bit, making it about 3.2 mm. I can easily explant a lens through that.”
- Consider using an existing wound to remove the old lens. “When removing a lens I just refresh the existing wound, just like you’d refresh a LASIK flap,” Dr. Grayson explains. “You can refresh a wound two or three months out and avoid cutting a new one. However, you don’t want to put a keratome into a previous wound, because inevitably you’ll create different levels and disrupt Descemet’s, which can get stuck in the new wound site if it has multiple levels.”
-
If the lens has been in the bag a long time, adjust your technique. “When you need to explant a lens that’s been in the bag for a long time, two techniques will help,” Dr. Rosenthal notes. “The first is to gently lift the edge of the capsule using a hypodermic needle or another fine instrument. One instrument that does this well is the LASIK flap lifter. The second technique is to use a combination of blunt dissection and viscodissection. You can get the capsular bag reopened in most cases using that approach.”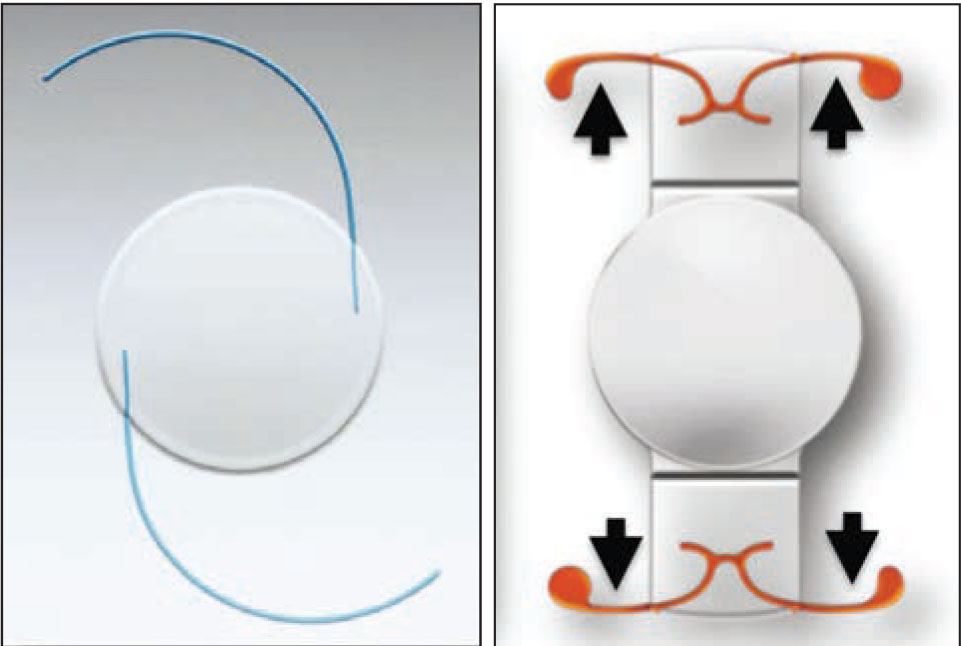
Lens and haptic design make a huge difference in how easy a lens is to explant after fibrosis has occurred. A loop-haptic lens (left) can usually be dialed out of the bag without a problem. The design of a Crystalens (right) makes it very difficult to explant once fibrosis occurs around the four haptics.
- Don’t be forceful when removing a lens. “You need to avoid pulling vigorously on the lens, because the globe may collapse,” says Dr. Rosenthal. “If that happens you may injure the corneal endothelium, not to mention cause retinal issues. You should just glide the lens out.”
Vitrectomy Tips
Although performing an anterior vitrectomy may not be a part of every lens exchange, it pays to be prepared to do it. Surgeons offer this advice:
- Make sure anterior vitrectomy is in your skillset. “I teach every resident that all anterior segment surgeons should have the ability to do an anterior vitrectomy through the pars plana,” says Dr. Rosenthal. “We teach a course on pars plana vitrectomy for the anterior segment surgeon at the ASCRS and Academy meetings, and residents these days typically learn this. It’s very important to be able to access the vitreous through the pars plana in conjunction with these techniques.”
- Know when to call in a vitreoretinal colleague. “If you’re only doing a limited vitrectomy because of a capsulotomy, it’s probably not necessary to have a vitreoretinal surgeon present,” says Dr. Rosenthal. “However, if the lens is completely dislocated and sitting on the optic nerve or the macula, and you have to bring it up in order to reposition it, someone trained in vitreoretinal surgery should be doing that.”
- When exchanging a lens in the presence of a capsulotomy, try visco-elevating the lens. “To do this, put some viscoelastic behind the lens, elevating it into the anterior chamber, injecting viscoelastic as you go,” explains Dr. Rosenthal. “The viscoelastic kind of plugs the capsulotomy, helping to prevent vitreous prolapse. I like to use Healon 5 for this because it’s a very retentive, viscous viscosurgical device, and it does a good job of plugging the capsulotomy. Once you’ve lifted the lens up you can implant the new lens underneath it, with the Healon 5 still in place. That helps to keep the bag maximally dilated, which is useful even if you decide to place the lens in the sulcus or use another method of fixation.”
- In the presence of a capsulotomy, only do a very limited vitrectomy. “This is a good idea for two reasons,” Dr. Rosenthal explains. “First, the purpose of doing a limited vitrectomy is to keep the anterior vitreous from prolapsing through the posterior capsule opening. You only need to remove a small amount of the anterior vitreous to accomplish this. The second thing is, you don’t want to do an extensive vitrectomy because you’re often going back into an eye with zonular or other support issues. You don’t want to compromise the vitreous humor completely, because it’s still helping to stabilize the capsular bag.”
- When doing a limited vitrectomy, stain the anterior vitreous using triamcinolone. “If you’re less experienced doing vitrectomy, putting a little triamcinolone in there will help you determine whether you’ve done enough,” explains Dr. Rosenthal. “Then, you just do enough vitrectomy so that you don’t see any triamcinolone, except behind the capsule. That tells you that you’ve done an adequate amount.”
- When doing a vitrectomy because of a capsulotomy, use trocars. “Rather than doing a one-time sclerotomy, I like to place pars plana trocars so I can go back in again if I need to do a little bit more vitrectomy,” explains Dr. Rosenthal. “This also gives me the option of using the vitrector to elevate the lens out of the bag. You can go in from behind and push it up.”
- If things don’t go well during the surgery, stop and come back later. “If a case is going south and you have to do a vitrectomy, you have retained nuclei, and the case was done under topical anesthesia and the patient’s getting restless, sometimes you have to just walk away from the table with no lens in the eye,” says Dr. Grayson. “The worst thing you can do is try to stuff in an AC lens or quickly put a lens in the sulcus. You can end up with iris prolapse, or an iris that’s shredded. That’s when those patients end up with long-term CME and corneal problems. Clean up as much as you can and then come back three or four weeks later when things cool down and the cornea’s clear and do your procedure.”
 |
| This patient’s previous radial keratotomy caused a hyperopic lens power error. The surgeon opted to exchange the lens rather than attempt a corneal laser correction over the RK incisions. |
Patient Management
In addition to managing patient expectations before surgery, you need to be prepared for situations in which the patient still isn’t happy, or cases where you choose to refer the patient to another surgeon.
Dr. Grayson points out that in some cases you may have to give premium patients their money back. “Sometimes when you replace a multifocal with a monofocal, the patient expects to get a refund,” he notes. “So, once you start taking premium lenses out, you have to be prepared to do something financially for the patient.
“If I have a patient who doesn’t adapt to a multifocal, and I’m taking the lens out and putting in a monofocal, I give them their money back for the multifocal lens,” he says. “The need to switch out the lens isn’t their fault or mine; the patient just turned out to be unable to adapt.”
Dr. Grayson also advises against referring a lens-exchange patient unless you have a good relationship with the surgeon you’re referring to. “If you’ve never done an exchange, or you only do one every few years, you might want to refer the patient,” he says. “However, you have to have a good relationship with the doctor you’re referring to. You can’t just tell the patient, ‘Go there and he’ll take care of you.’ It has to be a coordinated effort.
“Nobody wants to deal with an unhappy patient unless they have a good relationship with the referring doctor,” he explains. “If a patient just wanders in for a second opinion, and explains that the first doctor has told the patient he can’t do anything, I wouldn’t want to get involved. Among other things, if the patient needs a multifocal explanted and replaced with a monofocal, he or she may expect to get their money back. In that situation, I have to tell the patient to take that up with the original doctor. That has to be between the patient and the surgeon who put the lens in.”
Being Prepared
“My philosophy,” says Dr. Grayson, “is that if you want to be in the multifocal or post-refractive-surgery arena, you need to be able to do any modality of postop correction, including lens exchange. You can’t always say, ‘Oh, I’m going to do a PRK or LASIK touchup,’ especially in the post-refractive-surgery crowd. I look at a refractive touchup as a last resort, reserved for situations in which I’m not able to exchange the lens.
“I trained back in the 90s, when we were taught that if you put a lens in, it’s not coming out,” he says. “I think that philosophy is totally wrong. If you’re getting into the arena of refractive cataract surgery, and people are going to pay $3,000 or $4,000 for femtosecond multifocal visual improvement, you have to be able to fix any postop problems. You can’t just say, ‘Oh well, too bad. Wear glasses.’ ”
“I think it’s important to embrace the lens-exchange skill set,” he concludes. “People who feel they can teach themselves should do so. Watch videos online. Try parts of it during a regular cataract surgery; pop the lens out of the bag to see how it comes out. If you don’t want to develop those skills, that’s OK. However, in that case, you’ll need to develop a good relationship with another doctor who you can refer those patients to.”
Dr. Miller is a consultant for Alcon, BVI, Johnson & Johnson Surgical Vision, Long Bridge Medical and Oculus USA. Dr. Rosenthal is a consultant/KOL for Johnson & Johnson Vision. Drs. Grayson and Daluvoy report no financial ties to anything discussed in this article.