Though refractive surgeons are very aware of ectasia and the need to screen out risky corneas before eyes undergo LASIK, new indices and ways to analyze patients’ corneal data are constantly emerging. Here, experienced refractive surgeons and diagnosticians discuss the benefits and finer points of the methods they use for screening refractive surgery patients.
Heading Off the Problem
Experts are quick to remark that it doesn’t take much high technology to catch a patient with keratoconus—it’s detecting the ones with very subtle signs that takes the most sensitive and comprehensive instruments.
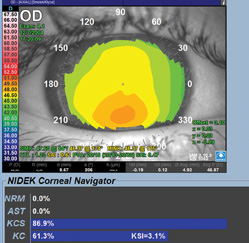
For Stephen Klyce, PhD, adjunct professor of ophthalmology at New York City’s Mt. Sinai School of Medicine, the key technology is corneal topography. “To show the subtle corneal surface changes that occur as a result of keratoconus, you need the accuracy and repeatability of placido topography,” he says. “With small changes in curvature, the placido mires become larger or smaller at a higher rate than the change that occurs in elevation between a steep and a flat area, so this sensitivity makes the change much easier to detect.”
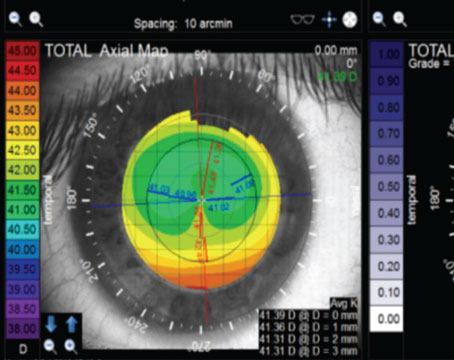
Albany, N.Y., surgeon Michael Belin prefers to use Scheimpflug imaging along the lines of that offered by the Oculus Pentacam. “For over 20 years now, I’ve been relying on elevation-based analysis,” he says. “Initially it was limited to the anterior surface on an elevation machine that dated back to the late 1980s, but now, for a little more than five years, I’ve relied on the Scheimpflug analysis of both the anterior and posterior surfaces and the full pachymetric map of the cornea it provides. I can’t imagine not evaluating the entire cornea when screening a patient. I believe the earliest keratoconic changes will occur either in terms of pachymetric progression changes or on the posterior surface. I want to pick up these changes early, and, for me, the most sensitive way to do that is to look at as many surfaces and as many parameters as possible.”
|
Rio de Janeiro’s Bruno Fontes, MD, PhD, says that, in his office, he has the Visante Omni combined topographer/optical coherence tomographer, the Pentacam and the Atlas topographer. “I combine the data from the Atlas placido with that from the Pentacam,” he says. “I think they’re great tools for detecting high-risk patients.” He’s also working on ways to get more accurate information from the Ocular Response Analyzer device.
Tips and Techniques
The experts say there are steps you can take in preparing the patient and using your diagnostic system that can enhance your screening process.
• Mind the ocular surface. To get the best data, you need a good image. “Make sure topography is the first thing you do,” says Dr. Klyce. “Don’t put dilating drops into the eyes first, because that can disturb the tear film and change the quality and/or the result of the image. Also, don’t have the patient sit there for 10 minutes with his eyes open, drying them out.”
• Watch your topographer’s scale. Using the incorrect scale to display the topography can give misleading results. “There are two important things regarding the scale,” says Dr. Klyce. “First, use a color palette with contrasting colors between each interval. And second, use a fixed scale. Whether you choose the standard scale of 1.5 D between colors or your own more sensitive one, such as a 0.5-D or 1-D scale, it should be fixed so you can recognize deviations between what a normal cornea looks like on your scale and an abnormal one.”
• Use the indices and screening tools of your device. “On the 10 or so indices I and my colleagues have developed for various topographers, we color-coded them after looking at the range, mean and standard deviation of normal patients with these indices,” avers Dr. Klyce. “If the index for a cornea you’re screening is two to three standard deviations away from the mean of normals, we color-code it yellow. If it’s more than three standard deviations away, it’s red. Usually, a particular index will steer you toward a specific abnormality.”
|
Dr. Belin thinks the posterior surface is a key to catching subtle keratoconus. “In the vast majority of patients I’ve seen, the magnitude of ectatic change in the posterior surface exceeds that of the anterior surface,” he says. “Or there’s pachymetric progression in spite of a normal anterior surface.” Dr. Fontes notes that the Visante Omni also has a global pachymetry map function similar to the Pentacam’s that can be useful for noting progression.
• Look at the big picture. Experts also take other factors into account. “Family history, ablation depth, asymmetry and the level of correction that you’ll be attempting all need to be considered,” says Dr. Belin. “If someone in his early 20s came in with a strong family history of keratoconus, that’s such a warning sign that I might not care what the imaging showed. Also, if there’s a high degree of asymmetry between the eyes, that’s a red flag to me.”
• Interpret the ORA carefully. Dr. Fontes says that though the ORA can give some helpful information, the way the data is presented can be a possible source of error. To acquire a reading, the ORA strikes the cornea with an air puff. It determines the corneal hysteresis value by subtracting P2 (the falling phase of the air jet) from P1 (the rising phase of the jet). However, P1 is always higher than P2 in any given eye, so it’s possible for a relatively weak cornea (with a low P1) or a stiff cornea (with a proportionately high P2) to have similar final low values for hysteresis, and therefore appear the same, even though one is healthy and one might be abnormal. As a possible solution to this, Dr. Fontes posits that it might be more helpful to look at the P1 and P2 as isolated values, study the area under the curve of corneal deformation and perform an analysis of the ORA waveform itself rather than rely solely on the CH and corneal resistance factor results.
In the future, Dr. Fontes foresees a time when surgeons will use biomechanical readings to modulate the laser correction. “If we have a very strong cornea and a very weak one, both with -1 D of myopia, for instance,” he says, “we could modulate the energy to give the same visual result in these two completely different patients.” REVIEW