Also in this Issue... Artificial Intelligence for the Cornea Specialist by Christine Yue Leonard DSO and Cultured Endothelial Cell Transplants: A Review by Thomas John, MD, and Anny M.S. Cheng, MD Diagnosis & Management of Blepharitis by Charles Bouchard, MD, MA |
Since I started implanting premium IOLs in 2002, I’ve learned when to offer these lenses and when to avoid them. To come to that decision, we evaluate the patient from front to back—from the ocular surface to macula—and address any findings with them preoperatively in order to avoid any mistrust or surprises postoperatively.
In this article we’ll focus on the anterior segment/cornea conditions that can affect the outcomes of premium IOLs, namely ocular surface disease, Salzmann’s nodular degeneration, epithelial basement membrane dystrophy, Fuchs’ endothelial dystrophy and irregular astigmatism.
Why the IOL Decision is Important
There are many reasons to avoid MF or even EDOF lenses in patients that have limited visual potential: (1) loss of contrast sensitivity from the IOL, (2) the perceived lack of value of a premium IOL if the patient is paying extra out-of-pocket and not achieving their best potential vision, (3) remove the confusion postoperatively as to the cause of decreased vision—i.e., is it the IOL or some other ocular pathology?
If there are any findings on the anterior segment or dilated fundus exam that will limit the patient’s visual potential, we typically recommend monofocal IOLs with or without monovision. By using monofocal IOLs we can provide our patients their best quality of vision possible.
Root Out the Cause of Poor Vision
First, we determine if the vision loss is from corneal pathology or internally (i.e., the lens). An easy way to determine if it’s a cornea or lens issue is by performing wavefront measurements (Tracey’s iTrace and Nidek’s OPD-III are common instruments available in the United States). We also use an RGP CL over-refraction in cases of irregular astigmatism and compare that to the manifest refraction; if the RGP over-refraction improves the BCVA then we know the cornea is the source of poor vision.
 |
|
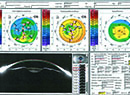
Figure 1. The image above shows the wavefront measurements of the eye and a combined placido disc image of the anterior corneal surface. The iTrace separates the total HOAs of the eye into the corneal and internal aberrations. The internal aberrations are assumed to be from the natural lens or IOL. In this case, the corneal aberrations make up the majority of the total HOAs (which is from corneal ectasia s/p LASIK) and therefore cataract or lens exchange surgery wouldn’t be beneficial for this patient; instead, the corneal aberrations should be addressed]. |
If we determine that the cornea is responsible for the loss in vision, we’ll focus on treating the corneal pathology first before recommending a lens implant. Figure 1 on page 17 shows an example of the cornea HOAs from corneal ectasia affecting the vision more than the internal HOAs.
Here’s a look at the main corneal causes of dissatisfaction with premium IOLs postop:
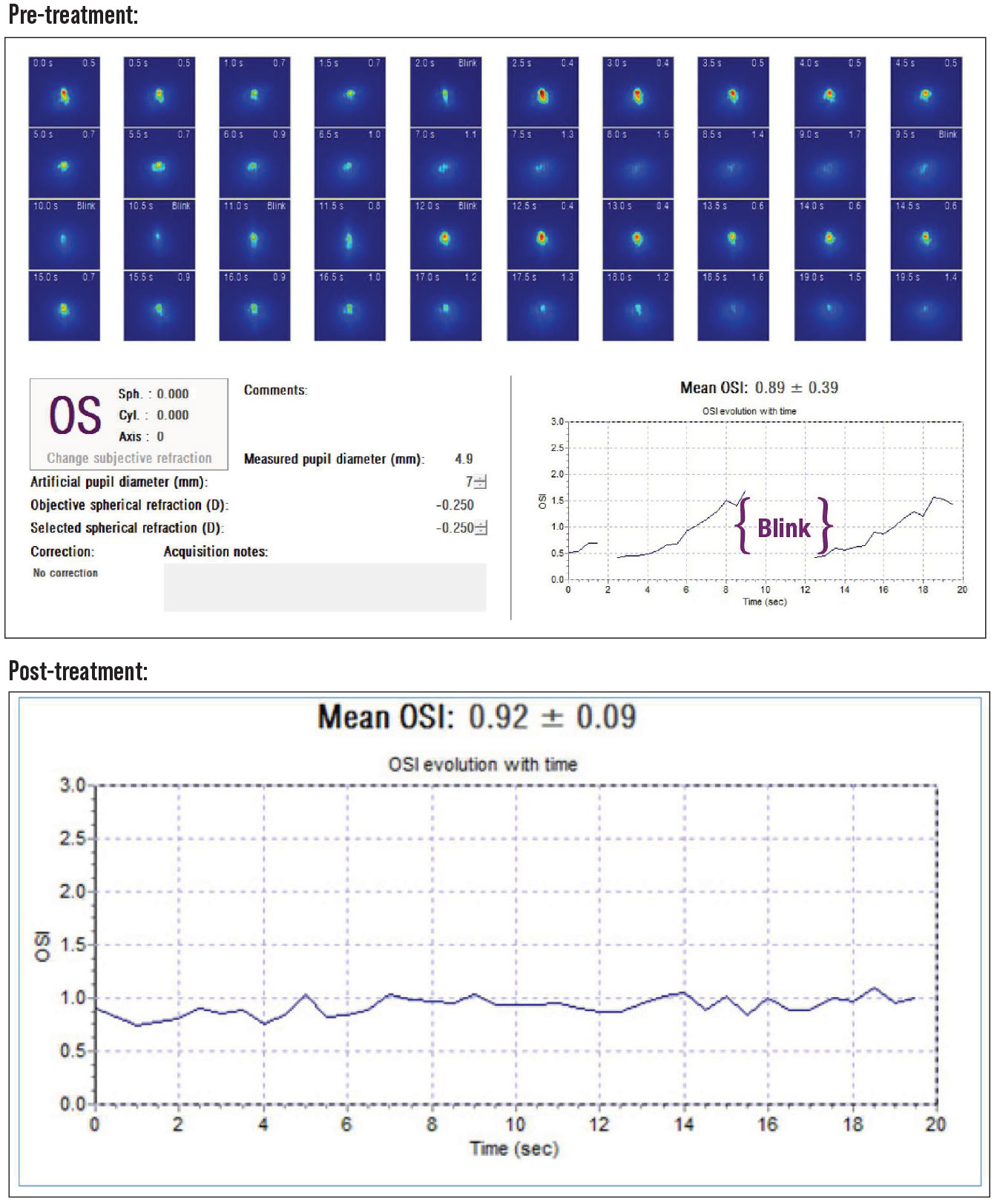
• Ocular surface disease. Dry eye is the most common corneal condition that must be addressed prior to recommending premium IOLs. Dry eye not only affects the quality of vision, but also the IOL calculations and the ability to perform enhancements for any residual refractive error. Dry eye can also alter the topography and A-scan measurements. An evaluation of the placido image on the topography, the keratometric image on the A-scan and the ocular scatter index (OSI) on the HD analyzer can help determine the clinical significance of the dry eye. If the images aren’t clear, we pretreat the patients with artificial tears, mild steroids and anti-inflammatory drops. We also recommend lid hygiene, and we still encourage oral omega-3 FAs. In severe cases, we recommend autologous serum drops. If the dry eye is significant enough to be a contraindication for LVC, we wouldn’t recommend any MF/EDOF lenses. Figure 2 shows an example of pre- and post-dry eye treatments on the OSI measurements.
• Epithelial basement membrane dystrophy and Salzmann’s nodular degeneration. These conditions affect the refractive outcome of surgery, limit LVC enhancement options and create HOAs/irregular astigmatism. If present, we recommend addressing these conditions preoperatively and allowing the vision and topography to stabilize before proceeding with lens implantation.
Our treatment approach includes initiating dry-eye treatment first and then performing superficial keratectomy with a diamond burr.
 |
|
Figure 2. Top: The OSI gradually increases as the patient focuses on a target without blinking, then once they blink the OSI improves before it increases as the patient focuses on the target again. This is consistent with an unstable tear film and lid margin disease. Bottom: After treatment, the OSI is low and stays low as they fixate on a target, showing a more stable tear film. |
The dry-eye treatment consists of artificial tears, lid hygiene, oral supplements (omega 3 FAs), and prescription dry-eye treatments. We don’t prefer one prescription dry-eye treatment over others; we typically start with what’s best covered by their health insurance and often use an independent pharmacy to assist us in this process.
Diamond burr superficial keratectomy for EBMD is performed under topical anesthesia in our laser suite or ASC. I like using a Took blade to test and remove all loose epithelium and then a diamond burr to gently treat the basement membrane. We then place a BCL soaked in a NSAID and sized appropriately to their K values, the postop management is the same as PRK/surface ablation treatments. SK for EBMD is curative, but recurrent corneal erosions can occur if all the abnormal basement membrane isn’t treated. Postoperatively, we wait until the ocular surface is normalized and the topography is stable and regular. Once they’re stable, we can then offer premium lenses and astigmatism treatment at the time of their cataract/refractive lens exchange surgery. Although LASIK can be offered once they are fully healed, I prefer surface ablations if an enhancement is needed.
For SND, the only difference in the procedure is finding the plane of the nodule(s), dissecting it off the Bowman’s layer and then using the diamond burr to create a smooth surface. Care is used to avoid removal of any stromal tissue. I also use MMC 0.02% for 30 seconds to reduce recurrence of nodules. SND patients typically also have significant dry eyes. Nodules can also recur over time even with the use of MMC and long-term dry-eye treatment. If you follow these patients postoperatively long enough, you’ll see continued OSD and changes in their refraction from the recurrence of nodules. LVC may also be contraindicated due to their dry-eye condition and irregular astigmatism. For these reasons, I rarely recommend premium IOLs in these patients.
A retrospective review of 20 consecutive EBMD cases discussed in the April 2010 Review of Ophthalmology article “The Benefits of Pretreating Corneas” found that the average change in MRSE was 0.64 D (range: 0 to 1.25 D of change). The average change in MRSE for SND was 1.7 D sphere (range: 0.5 to 6.5 D) and 1.57 D of cylinder (range: 0.25 to 4.5 D).
• Irregular astigmatism. This is another condition that can be a contraindication for a premium lens implant. Irregular astigmatism can be diagnosed by corneal topography and tomography. The most common causes include post-corneal refractive surgery ectasia (following LASIK, RK, PRK, etc.), keratoconus, pellucid marginal degeneration and corneal scars or opacities.
If the irregular astigmatism is caused by ectasia, one can’t confidently determine if the ectasia is stable or if it’ll progress. In these cases, I’ll review their past topographies and refractions and then, based on these findings as well as their age, history of eye rubbing and/or floppy eyelid conditions, make a surgical plan with the patient. If they’re older (over 40 for KCN, over 50 for PMD), I’ll consider a toric IOL. For post-surgical ectasias, however, I’m more hesitant.
 |
|
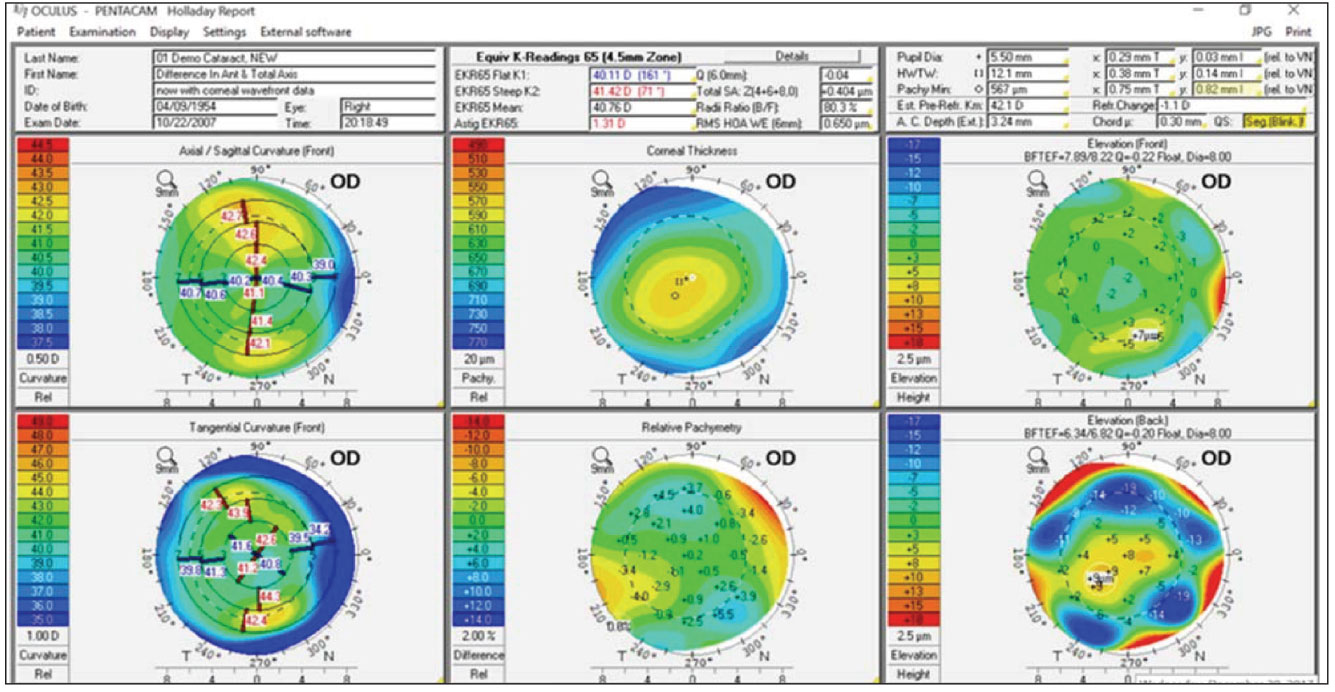
Figure 3. An example of the Holladay Report on the Oculus Pentacam showing the mean equivalent K reading for IOL calculations, total cornea HOAs, estimated refractive change from previous LVC, and chord µ values. |
For irregular astigmatism from injuries or infection, on the other hand, I’m more comfortable with toric lenses or even MF/EDOF lenses, as long as the patient’s corneal higher-order aberrations are low.
I especially like the Holladay cataract report on the Pentacam to determine if a patient is an appropriate candidate for premium lenses. This report shows the following, as described by the Holladay Report Interpretation Guidelines 2018 in the Pentacam manual:
- total spherical aberration to help pair the correct aspherical lens from 0 for hyperopic LVC to -0.27 for myopic LVC and RK;
- RMS (root mean square) HOA at 6 mm to measure corneal irregularity, if over 0.66 µm, a MF/EDOF lens may not be ideal, since the quality of vision may already be compromised;
- chord µ (analogous to angle kappa) to measure the chord distance from the corneal vertex to the pupil center; any value over 0.42 mm can cause worse uncorrected near vision and more night vision disturbances (glare and halos around lights); and
- predicted refractive change from previous cornea refractive surgery, to help with the IOL calculations. There are several other devices that we use in our clinic to measure the chord µ length, including the iTrace (Tracey Technologies), OPDIII (Marco Ophthalmic), and the IOLMaster 700 (Zeiss Meditec).
 |
|
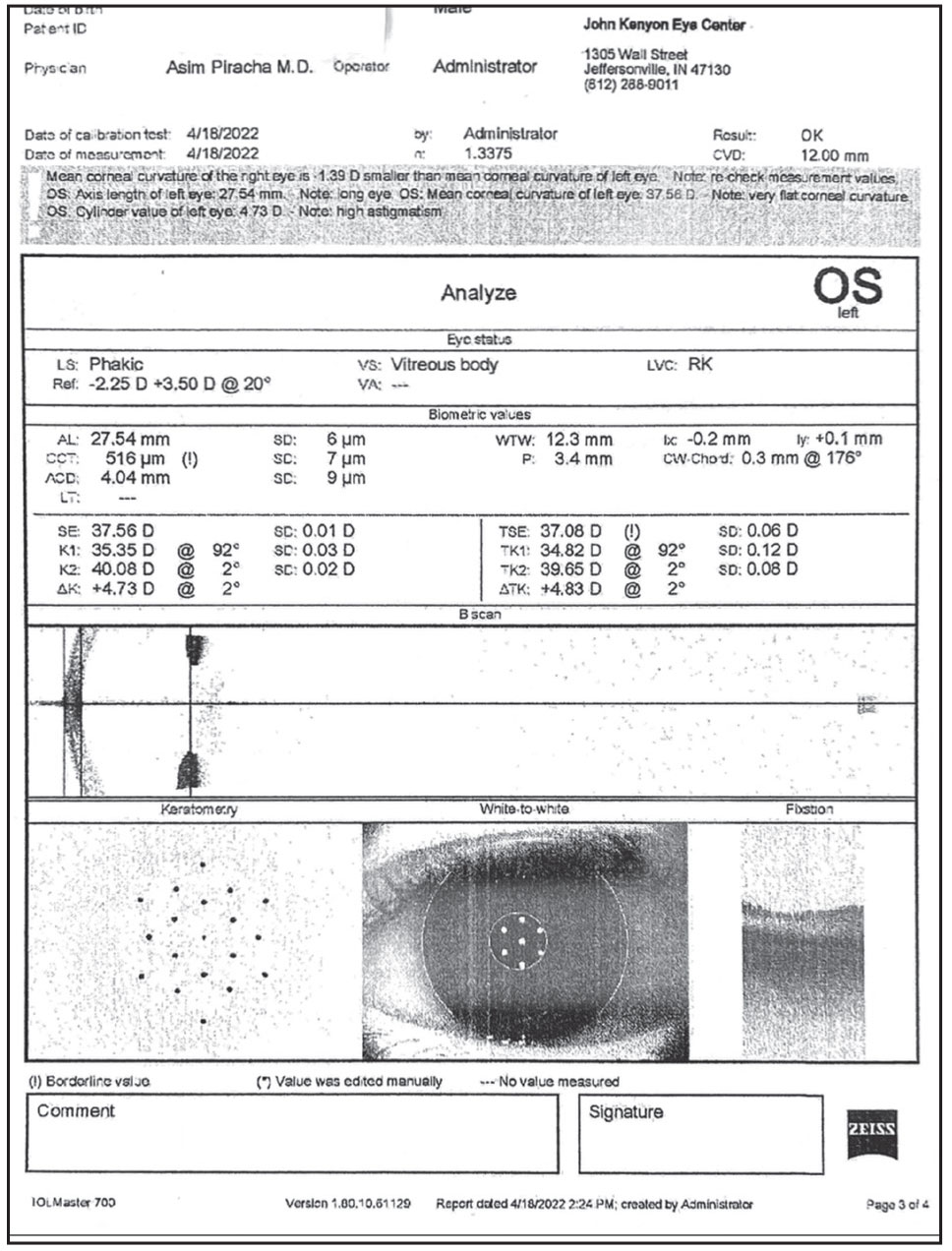
Figure 4. This is the IOLMaster 700’s analysis of data that includes the telemetric K values with image quality, visual axis and Chang-Waring chord µ value; and K and total K values with the amount and axis of astigmatism. In this case the TK measures 4.83 D at 2 degrees. |
If the RMS and chord µ values are outside of the normal range, I’m very cautious about using a MF lens. If the values are borderline and the patient understands how these findings can affect the performance of premium lenses, then I’m more comfortable with EDOF lenses like the Alcon Vivity, Johnson & Johnson Vision Symfony OptiBlue, or J&J Vision’s Eyhance.
I’ve been very happy with the quality of vision and function when pairing the Eyhance with previous myopic KRS. In patients with larger pupils, my experience is slightly better for the Symfony OptiBlue compared to the Alcon Vivity (a larger pupil affects the near function).
I’ve had success with toric lenses in patients with ectasia. If the ectasia is unstable, I’d recommend corneal cross-linking first and then a toric IOL once they’re stable after CXL. Some patients need more than CXL and benefit from intracorneal ring segments (ICRS) to reduce the corneal astigmatism and make it more regular.
• Fuchs’ endothelial corneal dystrophy. We also avoid MF IOLs in patients with Fuchs’ dystrophy. If the condition is clinically significant (i.e., diffuse central guttae and increased CCT) then we recommend a combined epithelial keratoplasty with phaco and implantation of a monofocal IOL. Even if the patient is relatively young (under 50) with mild guttae and normal CCT, we’re still very cautious with using a MF lens, since the condition can progress, it can affect the quality of vision and cause some refractive changes. I also prefer to avoid MF lenses in patients with Fuchs’ since the condition can progress after phaco or refractive lens exchange, limit their quality of vision due to the cornea, and limit the postop enhancement options.
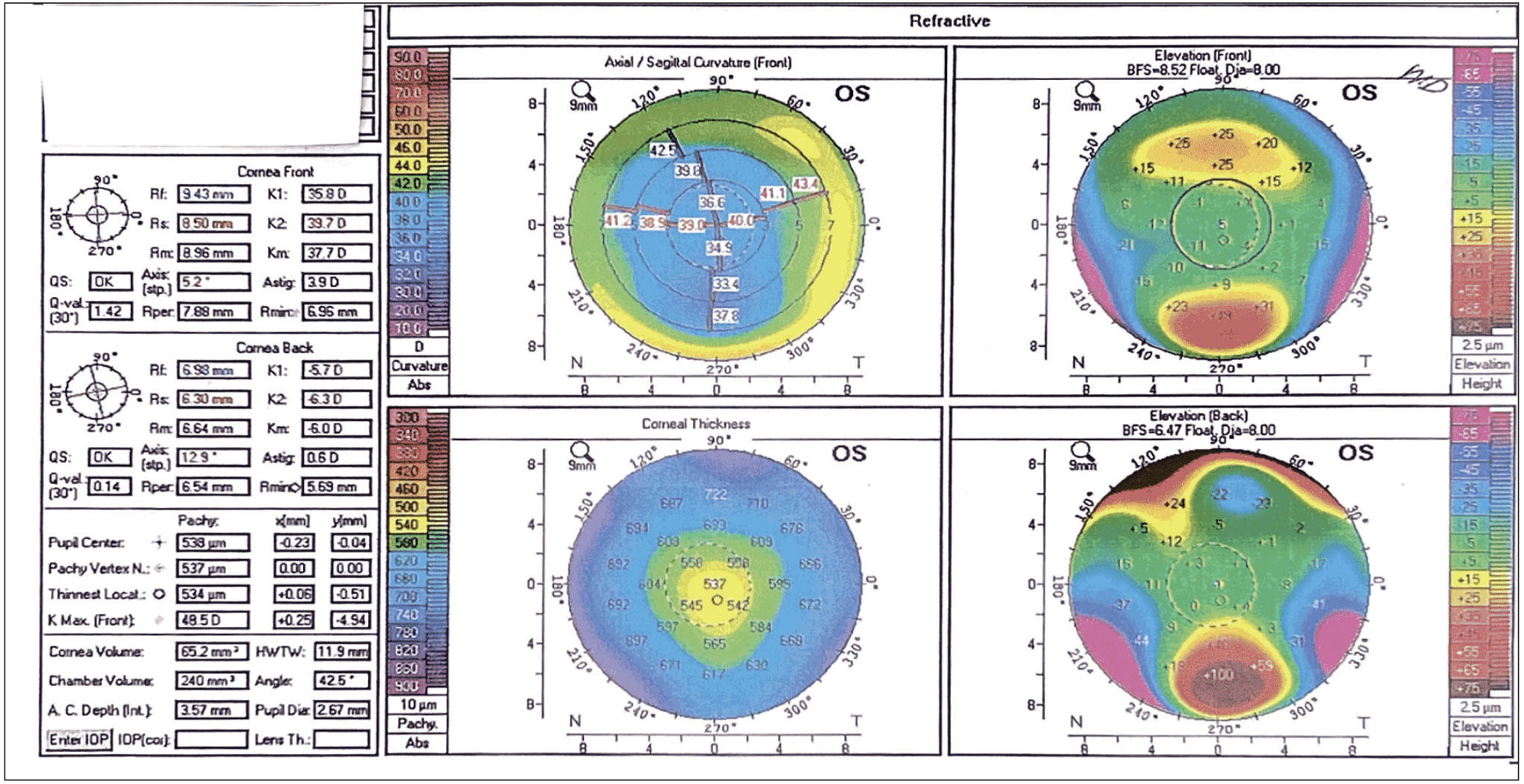 |
|
Figure 5. An Oculus 4 Map Refractive evaluation showing the front and back elevation, axial/sagittal curvature and central corneal thickness. The front curvature shows 3.9 D of astigmatism, with the steep meridian at 5.2 degrees. |
If a Fuchs’ patient is highly motivated to have premium lens, however, we’d consider an EDOF lens and perform DMEK to treat their condition. My experience with DMEK and DSEK (nano-thin, <60 µm), is that the corneal treatment can still induce an unpredictable refractive change. Although we target about -1 D for DSEK and -0.75 to -1 D for DMEK, we still frequently see outliers. In a recent case in which I performed a triple procedure (DMEK, phaco, IOL), the patient was +2.5 D MRSE and we targeted -1 D. When combining an EK with a premium IOL in this case, the patient will require a LVC enhancement, IOL exchange or a piggyback IOL.1,2 Note also that any intraocular surgery can damage the EK tissue and vision. Since this approach can be unpredictable and could take two or more surgeries to achieve good UCDVA, we recommend using monofocal aspheric IOLs and targeting good near or distance vision, but not both.
Case Study
The case is that of a 62 year-old male professional golfer, with previous RK surgery, cornea ectasia, BCVA of 20/50 and more than 4 D of irregular astigmatism (his images appear in Figures 4, 5 and 6). His topography and manifest refraction were stable, and he was very motivated to improve his UCVA and to reduce his dependence on glasses and contact lenses. After explaining that a toric IOL was off-label for him due to his irregular astigmatism, previous refractive surgery and the unpredictability of the refractive outcome, he was still very motivated to have toric lenses. His results were better than expected: UCVA 20/30+ and BCVA of 20/20. He was very pleased with the outcomes and is ready to get back on the tour.
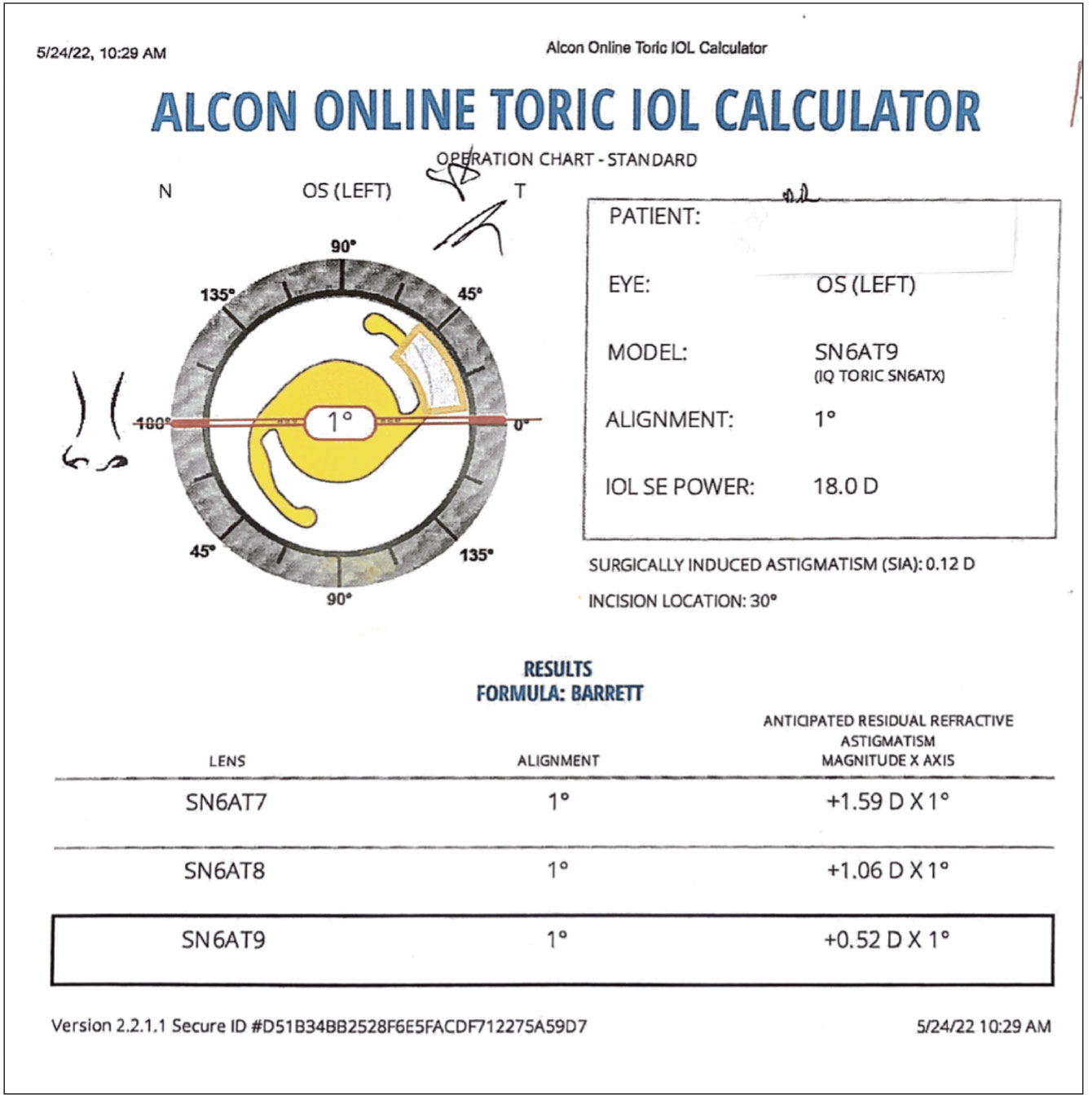 |
|
Figure 6. In this example, using the TK values, the Alcon Toric calculator recommends using the SN6AT9 (highest toric power) to treat the corneal astigmatism with an anticipated residual astigmatism of +0.52 D at 1 degree. |
In this example, using the TK values, the Alcon Toric calculator recommends using the SN6AT9 (highest toric power) to treat the corneal astigmatism with an anticipated residual astigmatism of +0.52 D at 1 degree.
Final Thoughts
So, can you use premium IOLs (specifically MF and EDOF lenses) in patients with pre-existing corneal pathology? The answer is … it depends. If the condition can be treated preoperatively to the point where the quality of vision isn’t affected by the cornea, the corneal topography/tomography is stable, there’s a low risk of the condition recurring or progressing, and they’re good candidates for LVC for enhancements after surgery, then the answer is “Yes.” The reason I’m so conservative with these cases is that if the refraction changes over time (as can occur with SND) the UCVA and BCVA will decrease, and the patient will be unhappy with their visual outcomes. What can add insult to injury for these patients is that there may not be a good surgical solution to improve their vision.
Since refractive surprises can occur with cataract and RLE surgery, the surgeon must be able to enhance them to correct any residual refractive error. Residual refractive errors with multifocal IOLs (bifocal or trifocal) can have a significant effect on their function for near, distance and low-light conditions. If a patient isn’t a candidate for LVC preoperatively for premium IOL surgery then, in my mind, they’re poor candidates for premium lens surgery too. Note that contraindications for LASIK include EBMD, SND, dry eyes and FCD. Advanced surface ablation is a better option in patients with clinically significant dry eyes or untreated FECD since interface fluid and poor flap adherence can occur with LASIK. LASIK, however, is an option for EBMD that’s responded to treatment.
Dr. Piracha is an associate professor at the University of Louisville’s Department of Ophthalmology and Visual Sciences.
1. van Dijk K, Ham L, Tse WHW, Liarakos VS, Quilendrino R, Yeh R-Y, Melles G. Near complete visual recovery and refractive stability in modern corneal transplantation: Descemet membrane endothelial keratoplasty (DMEK). Cont Lens Anterior Eye 2013;36:1:13-21.
2. Diener R, Treder M, Lauermann JL, Eter N, Alnawaiseh M. Optimizing intraocular lens power calculation using adjusted conventional keratometry for cataract surgery combined with Descemet membrane endothelial keratoplasty. Graefes Arch Clin Exp Ophthalmol 2022 Mar 8. doi: 10.1007/s00417-022-05598-6. Online ahead of print.
 |