The SMILE Procedure
The SMILE procedure uses the Visumax femtosecond laser to create an intrastromal refractive change in the cornea.
“In SMILE, the surgeon uses the Visumax to create a posterior refractive pass,” explains John Doan, MD, of St. Louis, one of the study’s principal investigators. “He then makes an anterior intrastromal pass that’s planar to the corneal surface. The posterior cut is a lenticular refractive cut and the anterior is simply the front surface of the lenticule, which has no refractive power. So, instead of vaporizing quarter-micron pieces of tissue, we’re creating a three-dimensional lenticule that we extract through this small incision. The removal of this lenticule induces central flattening in the cornea that treats the myopia. In that way, it’s somewhat analogous to the procedure automated lamellar keratoplasty, in which the surgeon made two passes with the microkeratome and removed a lenticule of tissue.” The incision that’s used to remove the lenticule is angled anywhere from 60 to 90 degrees. The lenticule itself is approximately 6.5 mm in diameter, and the outer dimension of the pocket that’s created is 8 mm.
“At the conclusion of the case, we remove the lenticule and spread it out on the anterior surface of the cornea to make sure there aren’t any missing pieces,” Dr. Doane explains. “If there are any, your only option at that point is to create a flap and look for the missing pieces, because you wouldn’t be able to see them otherwise.” The postop regimen is similar to LASIK’s, consisting of steroid and antibiotic drops for a week, with regular follow-up visits.
The Results
 |
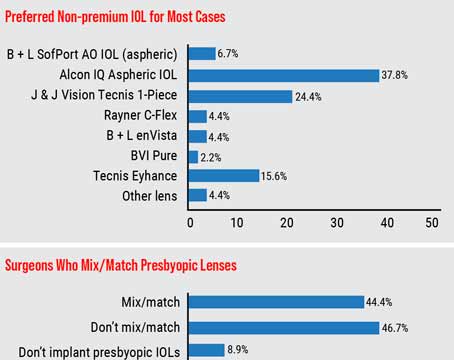
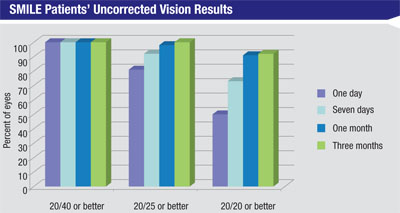
In the trial, patients had SMILE in one eye and LASIK in the other. In Dr. Doane’s 64 patients, the average sphere was -4.39 D (r: -1.5 to -7.5) and the average cylinder was -0.14 D (r: zero to -0.5 D). The average spherical equivalent refraction was -4.46 D (r: -1.63 to -7.75 D). In the 53 patients available for follow-up at a month, the average SE was -0.11 (r: -0.75 to +0.38 D), with the average sphere being zero and average astigmatism -0.21 D. For the 37 patients available at three months, the average SE was -0.06 D. All of the patients see 20/25 or better uncorrected postoperatively.
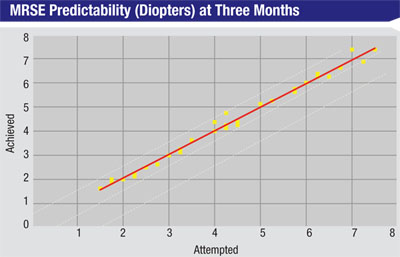
Dr. Doane says the treatment proved to be highly predictable. Using regression analysis, where an R2 value of 1 would mean perfect predictability between attempted and achieved refractions, in the SMILE study at three months the R2 value was 0.99. “What was interesting is that the predictability for 1 to 3 D treatments is just as good as LASIK,” Dr. Doane says. “However, for treatments of 6 to 8 D, LASIK’s predictability isn’t as good as it is for 1 to 3 D, so there’s a drop-off in how accurate we can be with the excimer. But this wasn’t the case with the SMILE procedure. So far its refractive predictability has been just as good with 6- to 8-D corrections as it is with the 1- to 3-D treatments.
“The patients have essentially been exactly where we want them to be in terms of refraction,” Dr. Doane continues. “Unlike the excimer, which needs to travel through the air before it hits the cornea and can therefore be affected by volatile substances such as particulates in the air or perfume, with the femtosecond we’re docked with the cornea, so it’s a completely closed system. The system is closed tighter still with SMILE because you’re doing the photodisruption within the cornea.”
In terms of safety, Dr. Doane says none of the eyes lost two lines of vision. “There is nothing new or out of the ordinary in terms of the incidence of complications associated with typical corneal refractive surgery,” he says. “There have been no infections and no epithelial ingrowth. There have been cases of mild diffuse lamellar keratitis in a few eyes as surgeons got used to the new technique, but nothing that’s led to lost lines of best-corrected vision. There can be an epithelial defect, which I’ve seen myself, but we also see that with LASIK.”
|
Looking down the road, Dr. Doane says Carl Zeiss Meditec is awaiting FDA approval to begin the final Phase III study of the device, and that the eventual approval may also involve compound myopic astigmatism in addition to just simple myopia. “Then we’ll have to see if we can treat mixed astigmatism and if we want to treat hyperopia,” he says. REVIEW