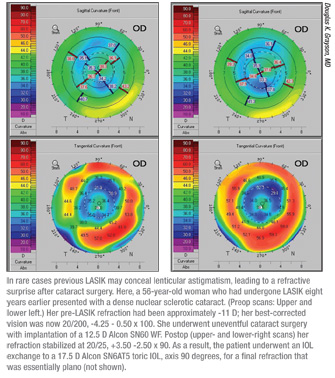
Here, five experienced surgeons share what they’ve learned about managing cases in which the patient has macular degeneration; has previously undergone laser refractive surgery; has undergone a corneal transplant; or has had radial keratotomy.
AMD: Should We Proceed?
Two fundamental concerns often arise when deciding whether or not to perform cataract surgery on a patient with macular degeneration: First, will the patient benefit enough from the cataract removal to make it worth the risks involved in surgery? And second, will the cataract surgery cause the retinal disease to worsen?
“It’s a misconception that patients who have macular degeneration will not benefit from cataract surgery,” says Tommy Korn, MD, FACS, who practices at Sharp Rees-Stealy Medi cal Group and is an attending ophthalmologist at Sharp Memorial Hos pital in San Diego.
“Patients with macular degeneration who’ve developed end-stage macular scarring are not blind; they still have functional peripheral vision. In these patients, the goal of cataract surgery is to improve the contrast sensitivity and quality of their peripheral vision.”
 Douglas K. Grayson, MD, medical director and chief of glaucoma and cataract surgery at Omni Eye Services in New York and New Jersey, also believes that even advanced macular degeneration patients may benefit from the removal of a dense cataract. “Removing a cataract may improve their peripheral vision and their navigating ability,” he says. “If they have a 2+ or 3+ nuclear sclerotic cataract and they’re 20/200 because of the macular degeneration, go ahead and make them 20/100. That’s worth doing. Ten or 15 years ago, the risk of cataract surgery was a big factor to consider in this situation, but it’s much safer today.”
Douglas K. Grayson, MD, medical director and chief of glaucoma and cataract surgery at Omni Eye Services in New York and New Jersey, also believes that even advanced macular degeneration patients may benefit from the removal of a dense cataract. “Removing a cataract may improve their peripheral vision and their navigating ability,” he says. “If they have a 2+ or 3+ nuclear sclerotic cataract and they’re 20/200 because of the macular degeneration, go ahead and make them 20/100. That’s worth doing. Ten or 15 years ago, the risk of cataract surgery was a big factor to consider in this situation, but it’s much safer today.”
The second concern—will the surgery make the macular degeneration worse?—is somewhat trickier. On the one hand, surgeons agree that operating on macular degeneration that’s already in the wet form is a bad idea. “The only way I would operate in the presence of active wet macular degeneration,” says Dr. Grayson, “is if the patient’s retina specialist said, ‘I want you to take out the cataract.’ Either the specialist’s view is compromised so that he can’t see where he’s sticking his Avastin needles any more, or he thinks this surgery will help, or he’s already got the disease under control. I would not proceed otherwise.”
On the other hand, if the patient has dry macular degeneration, the decision about whether to proceed with cataract surgery may be less clear-cut. “We know from the literature that a patient with dry AMD is not worsened by cataract surgery, but a patient who has an occult choroidal neovascular membrane can be harmed,” says Samuel Masket, MD, clinical professor of ophthalmology at the David Geffen School of Medicine, Jules Stein Eye Institute, at the University of California Los Angeles. “Historically, we’ve seen a number of eyes with previously undiagnosed wet AMD worsened by cataract surgery; it’s usually when the net is missed and not treated prior to cataract surgery. In all likelihood, the hypotony or varying IOP associated with surgery allows the subretinal neovascular membrane to expand and bleed or leak. For that reason, I go to great lengths to be certain that my patients with macular disease do not have any evidence of wet disease prior to surgery.”
Dr. Grayson agrees. “There haven’t been any conclusive studies showing that this can happen, but we do see it clinically, albeit rarely,” he says.
 |
 |
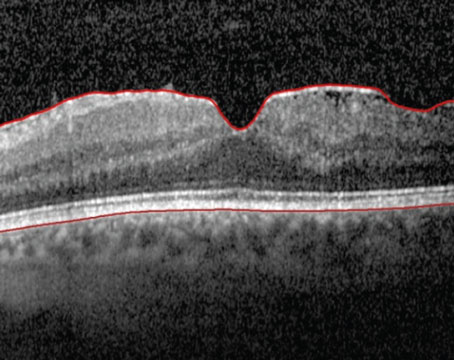
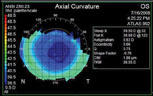
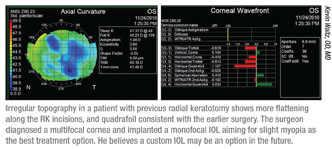
| A scan of a post-myopic LASIK patient reveals high, non-physiologic positive spherical aberrations consistent with myopic LASIK; other higher-order aberrations are minimal. The surgeon implanted a Tecnis lens to offset as much of the spherical aberration as possible. He anticipates that a custom IOL may be a reasonable option in the future.
| |
As noted by Dr. Skeens, an OCT scan is another preventive step many surgeons take, as is a fluorescein angiogram. “To avoid the possibility of leaking or bleeding, I think OCT is routinely indicated as part of the preop evaluation of a patient with macular degeneration,” says Dr. Masket.
Dr. Grayson agrees. “OCT might reveal significant thickening,” he says. “That would motivate me to have the patient see a retina specialist to get an angiogram and an assessment. I think a lot of those who get worse probably had occult neovascular membranes that were not clinically evident, but would have been angiographically evident.”
Dr. Masket also favors fluorescein angiography in these patients. “With a high-risk individual—someone who’s had a net, perhaps in the other eye, or has bled in the fellow eye following surgery—I insist that they have fluorescein angiography, usually within three to four days of the cataract surgery, to be certain that there’s no active lesion,” he says. “If we do find it, we ask the retina specialist to treat it. Then, after the patient’s macular region is dry, we’ll schedule his cataract surgery.”
Generally, an anti-VEGF drug such as bevacizumab is used to minimize any apparent risk of bleeding. “These days, if the patient has wet AMD, he gets a shot of Avastin a week or two before the surgery to keep everything under control during the postop period,” says Kevin Waltz, OD, MD, medical director of TLC Indiana, and assistant professor at the Indiana University School of Optometry. “I’ve been doing that for years and I haven’t had a single problem.”
AMD: Which Refractive Goal?
Because macular degeneration patients may have significantly reduced visual capacity, non-standard choices for the refractive goal are sometimes favored. However, opinions vary widely about what will help a patient in this situation the most:
• Plano. “I aim for plano with most of these patients,” says Dr. Grayson. “I know some surgeons like to aim to leave the patient more highly myopic when the patient has severe macular degeneration, to try to give him as much potential reading add as they can get. I believe we can manage that with spectacles or a low-vision aid. So, my goal is just to get them as clear as possible.”
• Slightly myopic. “At optics review courses I’m often asked: When a patient has end-stage macular degeneration, what should your refractive goal be?” says Dr. Korn. “I’d say it depends on the patient’s needs. Is the patient bedridden or active? Does he depend more on distance-related tasks, or near/intermediate tasks? I’d argue that if a patient has end-stage AMD and his livelihood is dependent on reading through the use of low-vision aids such as a magnifier or closed-circuit TV, it would be best to make him slightly myopic, around -2 or -3 D. When you do that, a 99-cent pair of over-the-counter reading glasses, around +2 or +3 D, instantly becomes a very inexpensive high-powered low-vision magnification aid. You also don’t have to grind the expensive prism seen in high-powered reading glasses if you make patients myopic after cataract surgery.”
• Hyperopic. “If you want to get adventurous, you can effectively create a Galilean telescope for the patient by choosing the IOL power to leave him hyperopic by 2 or 3 D and correcting this with spectacles,” says Dr. Waltz. “The result is a magnification effect that can be an effective visual aid for some of these patients. It’s easily done, especially if you’re a myope. If the patient is -8 D preop and has trouble with reading and you purposely leave him +2 D postop, he’ll get fantastic reading with that.
“On the other hand, if a patient is +6 preop with macular degeneration, and you leave him plano, he’ll be very unhappy,” he continues.
“You have to think about what the patient has become used to. If he starts out a high hyperope, he’s become accustomed to the magnification effect he gets with his glasses. If you leave him plano, you’ve taken away his magnification.
 “That’s probably one of the most common mistakes in ophthalmology,” he adds. “The surgeon corrects a high hyperope who has significant macular degeneration to plano and the patient is upset—and the ophthalmologist can’t understand why. The reality is that any dramatic change is likely to make this patient unhappy. So, in my experience, the best strategy is to leave these patients hyperopic. If the patient is +6 and has a compromised macula from macular degeneration, eliminate the cataract and leave him +5. He’ll be very happy. When he wears glasses, he’ll get the telescopic effect he’s accustomed to.”
“That’s probably one of the most common mistakes in ophthalmology,” he adds. “The surgeon corrects a high hyperope who has significant macular degeneration to plano and the patient is upset—and the ophthalmologist can’t understand why. The reality is that any dramatic change is likely to make this patient unhappy. So, in my experience, the best strategy is to leave these patients hyperopic. If the patient is +6 and has a compromised macula from macular degeneration, eliminate the cataract and leave him +5. He’ll be very happy. When he wears glasses, he’ll get the telescopic effect he’s accustomed to.”
Dr. Korn disagrees. “Although I’ve heard arguments for making these patients hyperopic, I don’t think that makes sense,” he says. “When you intentionally make a patient hyperopic, the retina never has a clear image focused on it; in contrast, if you make the patient myopic, he’ll have a clear image focused on the retina for near tasks. I think one of the best things you can do for macular degeneration patients is make them a little bit nearsighted.”
Regarding which type of IOL to implant, surgeons generally agree that a patient with macular degeneration is almost always a poor candidate for a multifocal lens, although an accommodative lens may sometimes work. “In mild, dry disease I have used accommodative lenses successfully,” notes Dr. Masket. “We want to do as much as possible to aid contrast in these patients, so we evaluate their preoperative corneal asphericity using the Marco 3D Wave. Then we use an aspheric IOL to try to match the patients’ asphericity.”
AMD Patient Expectations
Surgeons agree that in any non-routine cataract surgery situation, patient counseling is extremely important. One issue that patients need to understand is that there are at least two problems affecting their vision, so removing the cataract won’t give them back ideal vision.
 |
“The other helpful piece of evidence is the history,” she continues. “If the patient’s macular degeneration has been stable for a while, you can ask whether her vision has recently declined. Often the patient will say, ‘Yes, until a year ago I was okay, but things have gradually declined.’ That gradual decline is usually due to the cataract.
“Then,” she says, “you tell the patient, ‘You have macular degeneration, so I can’t fully predict what your vision will be like after cataract surgery. But based upon the symptoms you tell me you’ve been having with the cataract, as far as glare, gradual decline in your vision, and what your vision used to be compared to what it is now, I expect you to have vision improvement as a result of the cataract removal. We just won’t know the degree of improvement until the cataract is actually removed.’ ”
Another pitfall involving patient expectations is that visual acuity may not be as important to the patient as overall visual function. “Even though the patient may appreciate an improvement in acuity as we measure it on the chart, overall visual function may be a disappointment because of the reduction in contrast sensitivity from the macular disease,” notes Dr. Masket. “So patient counseling is key.”
A third issue is that the macular degeneration may worsen after cataract surgery, either in the short or long term. Dr. Korn notes that although it hasn’t been proven that cataract surgery can trigger choroidal neovascularization, it’s important to educate the patient about the difference between a cataract and macular degeneration, and the possibility that wet macular degeneration might develop—whether or not cataract surgery is performed.
| Being afraid can seriously undermine [a patient's] ability to pay attention and remember what you said.
—Kevin Waltz, OD, MD |
Dr. Waltz suggests one strategy that can play a crucial role in making sure your patient gets the message. “It’s ideal to include a family member in any discussion about the likely outcome of surgery,” he says. “Talking to the patient alone is not nearly as effective. Patients are often scared about having surgery, especially if they have macular degeneration, and being afraid can seriously undermine their ability to pay attention and remember what you said. Having a family member present means someone will definitely remember the discussion. So for your own protection and fairness to the patient, get a family member involved.”
Other Suggestions for AMD
Other Suggestions for AMD
Although most surgeons say they’d proceed with the surgery itself following normal protocols, a few additional strategies have been suggested:
• Avoid bright light during the surgery. “I’d keep the microscope light low to prevent any further damage to the macula, which is not healthy tissue,” advises Dr. Korn. “It makes no sense to potentially contribute to the problem by using bright light during the surgery.”
• Beware hypotony when wet disease has been curtailed. “If I take care of a patient who has been treated for wet macular disease, I make absolutely certain that the eye does not become hypotonous during the cataract procedure,” says Dr. Masket. “Hypotony may open up the choroidal neovascular membranes.”
• Postop, watch for choroidal membranes. “We watch these patients very carefully following surgery to make sure they haven’t developed a choroidal neovascular membrane,” notes Dr. Masket. “For that reason we see them a little bit more frequently, and we give them an Amsler grid, with instructions to report any change in its appearance.”
•Take extra steps to prevent postop edema. “If a patient with macular degeneration has significant macular changes postop, I’ll have him continue his nonsteroidal and steroid eyedrops for two to three months to help prevent macular edema,” says Dr. Skeens. “When patients don’t have a macular problem, I only keep them on those drops for one month.”
Post-Laser: Preop Concerns
As more cataract patients come in having already undergone refractive surgeries such as LASIK and PRK, issues surrounding their special needs and potential problems continue to increase in importance:
• Managing patient expectations. As with all non-routine cataract situations, these individuals need to have realistic expectations in order to prevent disappointment or anger afterwards. “These patients often require extra time and counseling,” observes Dr. Korn. “Many expect to end up seeing at all ranges without spectacles or contact lens correction, which is difficult even with the best IOL technology today. They also need to understand that we as ophthalmologists may have trouble predicting the correct power because the cornea has been altered. So I warn them that they may not be independent of spectacles, and that if the IOL power is completely off, they may end up needing either an enhancement or a lens exchange—assuming glasses or contact lenses don’t alleviate their symptoms. A Pentacam scan can provide useful data to help predict the correct lens power, but the surgeon still has to rely on other means such as old refractive records, RGP over-refraction analysis and experience.”
Dr. Grayson agrees that, given the difficulty of nailing the lens power, some of these patients will need further surgery to fine-tune the outcome. “There’s a significant chance that within four weeks we’ll have to go in and switch the lens,” he says. “We explain that to every patient.”
• Choosing the refractive goal. “When a cataract patient has had previous laser refractive surgery, I try to err on the side of myopia,” says Dr. Korn. “If you use standard keratometry or topography on these patients and use the power the computer says, they typically end up hyperopic. It’s always better to be myopic than hyperopic, because if you’re myopic, you still have a clear image up close. If you’re hyperopic, you never have a clear image.”
• Choosing the lens. One issue that often comes up with post-laser patients is whether they should be considered eligible for a multifocal or accommodative IOL. “I’m confident in offering these patients any lens that would make sense based on their lifestyle,” says Dr. Masket. “So, if the patient is otherwise a good candidate for a multifocal IOL, I will consider it.
“One of the concerns we have though, is making sure the patient had a good quality of vision prior to development of cataract,” he continues. “If the patient had a lot of issues with contrast or haloes and rings at night as a result of the laser vision correction, then you want to be very careful about IOL selection. I would definitely avoid multifocals, and I’d avoid an accommodative lens because the optic size is only 5 mm. On the other hand, if the patient was happy with his post-LASIK vision until the cataract evolved, I think he could be a candidate for any lens that would otherwise make sense.”
Dr. Masket notes one point in favor of trying a presbyopic lens in these patients: Post-laser patients have paid money to be spectacle-independent in the past. “They’re highly motivated and understand the situation,” he notes. “As long as they’re willing to have enhancements or exchanges as necessary, then I’m willing to help them achieve spectacle independence. The exception would be if their previous laser correction was for monovision and they liked it. In that situation, my approach would be monovision. It makes sense to stay with a winner.”
 |
“Because spherical aberration occurs in proportion to the amount of pupillary aperture, the other thing you can do is constrict the pupil with pilocarpine,” he continues. “Or, you can purposely leave the capsulorhexis smaller than you normally would. Most surgeons would make the capsulorhexis about 5 or 5.5 mm in diameter; if you make it 4 or 4.5 mm, it acts as an occluder after it heals and opacifies, cutting down on the effect of spherical aberration. This strategy will work for both hyperopes and myopes, and the higher the original correction, the more important these modifications become.”
Post-laser Surgical Issues
The fact that the cornea has been altered raises a number of intraoperative concerns:
• Incision location. Dr. Grayson notes that a clear corneal wound can induce astigmatic error in previous LASIK patients. “Some people advocate doing a scleral tunnel in previous LASIK patients,” he notes. “How- ever, I still believe that you have the best surgical access operating temporally, and it’s not good to do a scleral tunnel temporally; the patient feels it, it’s uncomfortable for the surgeon, and the conjunctiva retracts. So, I stay with the clear corneal incision. But I make sure to stay as far peripheral as I can so I don’t cross the interface of the LASIK button, which can induce a lot of astigmatic error. Sometimes I make a bit of a limbal incision to remove the conjunctiva, just so I can get as far back as possible into peripheral clear cornea. I also use a 2.2-mm wound, which I think is an asset in those patients.”
• Refraction vs. anatomy. Dr. Korn notes that the refraction may not reflect the anatomy of the eye. “These patients were typically high myopes before their laser surgery,” he points out. “The surgeon needs to remember that even though the current refraction is not myopic, the eyeball is still myopic. As a result, when you do the cataract surgery the anterior chamber has a tendency to deepen when the irrigating fluid enters the eye. For that reason, I’d make some bottle height adjustments in patients who were severely myopic in the past.”
• Masked lenticular astigmatism. The fact that the cornea has already undergone surgery may occasionally lead to situations that would not be encountered in a virgin eye. For example, Dr. Grayson notes that previous LASIK may, in some cases, mask astigmatism in the crystalline lens. “We had a previous LASIK patient whose manifest refraction didn’t show any astigmatic error,” he says. “I implanted a monofocal lens, and found that she was suddenly manifesting 2.5 D of cylinder. (See scans, p. 48.) It happened that the power was wrong anyway because of the difficulty of power calculation after LASIK, so I exchanged the monofocal for a toric, aligning her axis with the refraction rather than trying to align it using the keratometry. That worked out perfectly.”
• Off-center surgery. “Higher-order aberrations will increase overall if the earlier treatment was decentered,” Dr. Waltz points out.
“Topography will show you whether this is the case. If the earlier treatment was decentered, you’ll have to talk to the patient; he may need a rigid gas-permeable contact lens to fix the problem, because it’s not easily fixable with surgery. The only thing you can do is make the capsulotomy smaller, but that will have limited beneficial effect in this situation; RPGs are much more effective. Patients may not be thrilled about that, so you want to have the discussion before surgery, not after.”
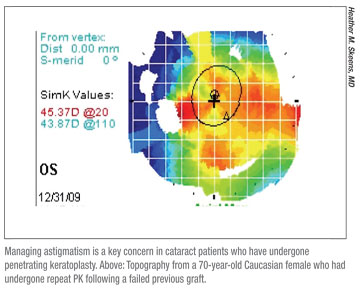
Post PK: Managing Astigmatism
“Topography will show you whether this is the case. If the earlier treatment was decentered, you’ll have to talk to the patient; he may need a rigid gas-permeable contact lens to fix the problem, because it’s not easily fixable with surgery. The only thing you can do is make the capsulotomy smaller, but that will have limited beneficial effect in this situation; RPGs are much more effective. Patients may not be thrilled about that, so you want to have the discussion before surgery, not after.”
Post PK: Managing Astigmatism
“The number-one issue with post-PK patients is cylinder,” says Dr. Waltz. “Penetrating keratoplasty surgeons are often thrilled if a patient only ends up with 3 D of residual astigmatism, so you should expect to encounter significant cylinder in these patients.”
Some surgeons prefer addressing this surgically. “If the PK patient has astigmatism in the graft at the time of cataract surgery, you can always place relaxing incisions in the graft to correct it,” says Dr. Skeens. “If the patient has residual unwanted astigmatism in the graft after surgery, I might perform a refractive procedure over the graft, such as PRK. I prefer not to use LASIK in these cases, because I like to avoid cutting a flap, due to the slight risk of graft dehiscence.”
The other option—which has found increasing favor in virgin eyes—is a toric lens. Surgeons are hesitant to apply that solution in a post-PK eye, although opinions differ to some extent. “If the cylinder is regular, you can treat it with a toric lens,” says Dr. Waltz. “If it’s not, you’re going to have a hard time doing much about it. Most patients in this situation will have to wear rigid contact lenses postop to get the best vision.”
“If the patient has a degree of regular astigmatism and his graft is in good health—it’s not particularly thick, the endothelial cell counts are reasonably good, the morphology of the cells is reasonably good, and we expect graft survival—the patient might be good candidate for a toric lens,” agrees Dr. Masket.
Dr. Korn is very cautious about using a toric lens under these conditions. “There are two components to astigmatism: corneal and lenticular,” he notes. “Toric lens implants introduce lenticular astigmatism that negates corneal astigmatism. But if refractive corneal irregularity is caused by the transplant or suturing technique, the toric lens won’t correct it. I’ve had contact lens specialists tell me that it can be nearly impossible to refract these [toric implant] patients to 20/20 because they have components of both lenticular astigmatism and corneal astigmatism.” Dr. Korn says he might make an exception if the post-PK patient’s topography shows only regular astigmatism and no evidence of irregular astigmatism. “But even in that case the surgeon has to be careful about inducing additional astigmatism at the time of cataract surgery,” he points out.
Dr. Skeens favors avoiding a toric in this situation. “I don’t think these patients are good candidates for a toric IOL, because if anything happens to the graft—if the patient ever needs a repeat graft, or has an injury to the eye, or if something causes the graft to shift—then the toric IOL will no longer be accurate. So I would just use a basic monofocal lens.”
Dr. Masket notes that these future risks may be less of an issue with the advent of procedures such as Descemet’s stripping endothelial keratoplasty. “In some patients, the graft may be at risk for decompensation and a regraft, “ he says, “but a failed penetrating keratoplasty can be replaced by a DSEK, which is very unlikely to induce significantly more astigmatism. Given that reality, I think a toric lens would probably be the lens of choice—assuming there’s an appropriate amount of regular or near-regular astigmatism.”
Dr. Korn adds that it’s important to know if the patient is already using a contact lens to correct corneal astigmatism. “If that’s the case, it’s probably best to implant a monofocal IOL, because an RGP contact lens can eliminate significant amounts of both regular and irregular corneal astigmatism,” he says. “A toric IOL can only eliminate regular corneal astigmatism.”
Dr. Waltz adds a cautionary thought: “These are the kind of cases that a really experienced corneal specialist needs to see,” he says. “There aren’t enough PKs coming into most practices for a surgeon to get good at managing them. They can present very complex optical issues.”
Other Post-PK Strategies
These protocols can also increase your chances of a good outcome:
• Manage any dry eye. “Penetrating keratoplasty patients all have problems with dry eye,” notes Dr. Waltz. “Paying attention to dry-eye treatment before surgery will pay big dividends after the surgery.”
• Make sure the corneal curva-ture has stabilized. “To get an accurate power for the lens implant, the astigmatism must have stabilized and be acceptable,” says Dr. Skeens, noting that this depends partly on how recently the PK was done. “If the PK is recent, you need to follow the corneal keratometry values and work with suture removal to minimize postoperative astigmatism, prior to cataract removal.“
“In our practice, we wouldn’t perform cataract surgery on a PK patient until about a year out, to allow the corneal incision to heal,” notes Dr. Korn. “To ensure that the keratometry is stable, we like to wait until a couple of successive readings show that the topography and keratometry are consistent and reproducible.”
• Avoid silicone implants. “Eyes that have had a previous transplant are typically sick eyes; they may have coexisting, comorbid conditions such as retinal disease,” notes Dr. Korn. “If you implant a silicone intraocular lens and the patient subsequently has a retinal detachment, the retina surgeon will have difficulty repairing the eye. Oil and gas typically condense on silicone implants during retina repair surgery. A good retina surgeon is a cataract surgeon’s best friend, so I don’t want to make his life miserable when he’s repairing the retina.”
• Be wary of using a presbyopic lens. “The corneal surface is much different after a penetrating keratoplasty,” says Dr. Korn. “There may be isolated areas of irregular astigmatism. To introduce a multifocal IOL, with optical properties that scatter light, into a corneal transplant eye opens up the possibility of visual problems that can result in loss of best corrected acuity and contrast—not to mention generating bothersome halo and glare. In my experience, these patients do better with a monofocal implant.”
“If the patient wears a contact lens to manage post-graft astigmatism and has good quality of vision, he theoretically could have a multifocal,” says Dr. Masket. “However, I think that would be very atypical. Also, you’d need to consider whether the indication for the PK was keratoconus, which can recur in the grafted patient, even if the patient has done well for several years. Given that possibility, I’d stay away from any type of presbyopic lens in such a patient. I’d probably use an aspheric lens, but definitely single-vision.”
• Avoid causing corneal trauma. “With penetrating keratoplasty patients, the most important concern is to avoid damaging the corneal tissue,” says Dr. Korn. “To protect the cornea, I prefer to make a scleral or limbal incision rather than a clear cornea incision, to help ensure incision stability. A scleral tunnel does take longer, but I would rather err on the side of safety. Also, intraoperatively, I might use a more dispersive viscoelastic to protect the corneal endothelium.”
“The incisional track should be relatively short,” says Dr. Masket. “And in this situation, even if I make a short track, I’m going to use sutures to close the incision. In fact, some surgeons would recommend doing a sclero-corneal tunnel rather than a corneal tunnel—but I don’t necessarily share that opinion.
“These patients typically have a low endothelial cell count,” he continues, “so you must pay attention to the endothelium and be protective. During the surgery I typically lower the infusion bottle to reduce the amount of turbulence. I use a slower turnover—what Robert Osher, MD, refers to as ‘slow-motion phaco’—and I repeatedly instill a retentive viscoelastic agent, preferably Viscoat. I also try to disassemble the lens as much as I can within the confines of the capsule bag. I don’t recommend doing a phaco flip or any other procedure in which the nucleus is brought into the chamber—you want to stay as far away from the endothelium as you can.”
Noting that these patients are also vulnerable to an inflammatory/immune response, Dr. Masket suggests that they be covered heavily with steroids prior to surgery and later on in the perioperative period. “In this situation,” he says, “I might suggest the use of Durezol, a very potent steroid.”
“When the surgery is completed, I check for leaks by doing fluorescein staining over the host-donor cornea junction site,” notes Dr. Korn. “Beyond that, it’s very important that these eyes be followed a little more closely postop than the routine cataract patient. This is not only to make sure there’s no infection or inflammation, but more importantly to make sure that there’s no sign of corneal graft rejection, and also that the corneal incision and the host/graft interface is still structurally secure. Cataract surgery can be a stressful event for the eye, and could possibly trigger a rejection episode in a patient with a corneal transplant.”
Post-RK: Patient Expectations
Given the complex nature of a post-RK cornea, patients need to understand several potential concerns that could affect their outcome:
• Power calculation challenges. “The post-RK patient tends to have progressive corneal flattening, creating a moving target in IOP power,” Dr. Masket points out. “And, they tend to fluctuate over the course of the day; the cornea tends to be flatter in the morning and steeper in the evening. So it’s very difficult to nail a refractive outcome for the RK patient, even if we can measure them properly. Patients need to be aware first, of the difficulty of calculating power; and second, of the fact that even if we make their vision perfect at 10 in the morning, it may not be right at 10 in the evening.”
• The cornea will take time to stabilize. “A post-RK cornea always flattens as a result of cataract surgery,” says Dr. Waltz. “This lasts for a couple of months and then goes away. For example, let’s say you’re aiming for plano on an RK eye. At the first week, you should expect the eye to be +1 or +2 D. If you achieve plano in the first week, the patient will end up myopic. The exact amount isn’t predictable, but the fact that it will happen is predictable. You need to have a discussion with the patient so he understands that he’s not going to see the final result for two or three months.”
• Possible long-term postop refractive shift. Dr. Skeens notes that one concern with a former RK patient is the tendency for RK eyes to have a progressive flattening of the cornea that induces hyperopia over a period of years. “If you’re dealing with someone who hasn’t been your patient and who has no previous records, you may not know if the cornea has been stable or is progressively getting worse,” she points out. “So, tell the patient she may still have to wear glasses or contacts after surgery. You’ll do your best to keep her out of them. But even if she doesn’t need them right away, she could still need to wear them later on.”
Post-RK: Which Refractive Goal?
When dealing with a cornea that’s undergone RK, a number of factors should be considered when determining the best refractive outcome:
• Factor in the number of RK incisions. Dr. Masket says he alters his IOL power calculation based upon the number of incisions the patient has had and how small the optical zone is. “A patient who has had as few as four incisions may need very little adjustment to IOL power calculation. On the other hand, a 16-cut out to the limbus and a 2.5-mm optical zone will require significant adjustment,” he explains. “I add anywhere from 0.5 D up to 2 or 2.5 D of IOL power to what we’ve calculated, based upon the number and length of incisions.”
• Compensate for measuring a small optical zone. “The biggest problem is getting a good central corneal reading,” notes Dr. Masket. “The cornea always tends to be flatter than we find by keratometry, in part because standard keratometers may read a 4-mm optical zone. The IOLMaster reads a 2.5-mm zone, which is a little bit more accurate, so we measure the post-RK cornea with the IOLMaster.” (Dr. Masket says he has begun using the LenStar in his practice, but hasn’t yet tried it with a post-RK patient.) “We measure the RK cornea topographically and keratometrically, and take the flattest readings we can and enter those into the formula,” he adds.
• Consider the impact of location and altitude. Dr. Korn notes that RK eyes may undergo refractive changes at different altitudes, so the location of the surgery and the patient’s home matter. “It’s important to find out whether the patient will be living at a different altitude than where the surgery is taking place,” he points out. “That may alter the final lens prescription.”
• Be cautious about aiming for a myopic result. Because RK eyes may shift toward hyperopia over time, some surgeons deliberately aim to leave the patient slightly myopic after cataract surgery. Dr. Skeens says she doesn’t routinely do this. “The problem is that you don’t know for certain that they will experience a progressive hyperopic shift following cataract surgery. Furthermore, even if a hyperopic shift occurs, you don’t know how much shift there will be, so you don’t know how myopic to leave them.”
RK: Choosing the Right Lens
An RK eye can make some types of implant more problematic:
• Multifocals. Surgeons see several reasons to avoid implanting a multifocal in a post-RK patient. “An RK eye has many areas of irregular astigmatism, so it wouldn’t be prudent to implant an IOL that induces halo and glare; the patient is already likely to be suffering from halo and glare because of the RK incisions,” says Dr. Korn.
Dr. Waltz adds that because of the RK, the prescription postop is going to be unstable on a daily diurnal basis. “That will be in proportion to how much RK treatment was done,” he says. “So you can’t count on the patient having the same prescription at 9 in the morning and 9 at night. Under those conditions, a multifocal lens is less than ideal.”
• Accommodating lens. Some sur- geons are willing to consider implanting a Crystalens. “I don’t have an objection to the use of accommodating lenses, since they don’t reduce contrast sensitivity,” says Dr. Masket. “However, I think patients may be disappointed in their postop results. After all, they’re spending significant out-of-pocket money for these devices. Nevertheless, if the patient understands this, is highly motivated and is willing to risk the money for an accommodating lens, I don’t see a contraindication to using it.”
Dr. Waltz points out that the multifocality of a post-RK cornea can actually be used to your advantage. “The patient will have more flexibility of vision with a monofocal or Crystalens—assuming that you understand how the patient’s vision changes each day over the diurnal curve,” he says. “In fact, the Crystalens is the one presbyopic lens that may work for an RK patient—if the patient is really motivated to be less dependent on glasses. However, because these patients are paying extra and they’ve already had RK, their expectations are quite high. You have to have a tho-rough discussion with them ahead of time to make sure they understand that this is not going to produce perfect vision—although it may make their vision better than it would have been otherwise.”
• Toric lens. “I’ve been both successful and unsuccessful using toric lenses in post-RK patients,” says Dr. Masket. “So, patient counseling is very important. If the astigmatism ap- pears to be regular and it’s within the parameters of the available toric lenses, I will use one—as long as the patient understands that his astigmatism may change postop. I don’t have a contraindication to using them; patients just need to be prepared for possible disappointment.”
“If an RK patient has regular astigmatism, it would be reasonable to try to put a toric lens in,” agrees Dr. Waltz. “However, most RK patients don’t have a lot of astigmatism, despite their irregular corneas. RK wasn’t a very effective treatment for astigmatism, so most RK surgeons tended to eliminate people with a lot of astigmatism from the patient pool.”
Post-RK Surgical Concerns
Post-RK patients can benefit from these strategies:
• Determine the source of the vision loss. Dr. Skeens notes that it’s important to be sure the cataract is really the problem. “You have to explain to the patient that part of the visual decline may be from an RK-related progression in the cornea,” she says. “If the individual has a very mild cataract, and you’re not sure whether the cataract is really causing the vision loss, then you might decide to follow the patient’s cornea for a while.“
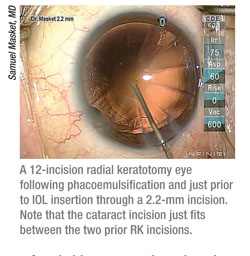
• Choose your incision location judiciously. “It’s important to avoid stressing the RK incisions, and obviously, to avoid cutting across them,” says Dr. Masket. “Today’s microincisions help with that; my standard corneal incision is 2.2 mm. I can go in through fresh, undisturbed tissue and not open the previous incisions, even in a cornea that’s had an eight-cut RK. However, when you get to 12 or 16 incisions, you have to resort to a sclero-corneal approach; and it has to be made more posteriorly to avoid intersecting with the old keratotomy incisions. If you do disturb them, you’ll have to close them, and you’ll create additional irregular astigmatism that can take months to resolve.”
Dr. Korn says he always uses a scleral tunnel incision in these patients. “If you make a clear corneal incision close to the RK incision and it splits open when you raise the bottle height—which I’ve seen happen—then you have to close the incision. And by closing it, you’ve altered the refractive power and curvature of the cornea, which means the IOL power you selected will be completely off. For that reason, I always use a scleral tunnel incision and avoid the cornea, even if the number of RK in-cisions was small.”
Dr. Korn adds that with RK patients he prefers to suture the cataract incisions closed, whether they are scleral tunnel or corneal incisions. “These eyes can have poor wound stability,” he explains. “They’re at risk of developing a wound leak after surgery.”
• Minimize your impact on the RK incisions. “With a high infusion pressure over a long period of time, you’ll create greater instability in the RK incisions,” says Dr. Masket. “I try to reduce that by lowering the height of the bottle and being in the eye as short a period of time as possible.”
Dr. Waltz agrees. “The goal when making the RK incisions was to get a 90-percent-depth cut,” he points out. “That’s cutting it fine, and sometimes perforations resulted. A small perforation during the surgery was called a micro-perf, which self-sealed; a macro-perf was one you had to suture. When you perform cataract sur- gery on one of these individuals there’s visible evidence if one of the RK cuts had a suture—but you won’t see any evidence of a micro-perf. So you don’t know exactly what you may be dealing with, and when you hyperinflate the eye during cataract surgery, sometimes the incisions will leak. It’s advisable to lower your bottle height to discourage that from happening.”
• Don’t make postop adjustments until the cornea stabilizes. “Postoperatively, we measure corneal curvature at every visit, and we typically see some transient flattening,” says Dr. Masket. “You have to wait until the cornea returns to its preop keratometric readings to prescribe glasses, contacts, or do enhancements or exchanges, if they are necessary. At that point there is a stable situation.” Dr. Masket says he’s seen as much as 3 D of flattening occur postop, and take as long as three months to go away—although he says that was before he modified some parts of his surgical technique. ”Now that we use a low infusion bottle and do the procedure as quickly and expeditiously as possible, we don’t tend to see that much fluctuation anymore,” he notes. “But I still see some fluctuation, and the patient must be made aware of it.”
Drs. Korn and Grayson have no financial ties to any product mentioned in this article. Dr. Masket is a consultant to Alcon Labs and receives speaking honoraria from Bausch + Lomb. Dr. Skeens consults for Bausch + Lomb regarding eye- drop products. Dr. Waltz is a consultant to AMO.