The Roots of Stem Cells
Stem cell research dates back to the early 20th century, but it wasn’t until 1981 that embryonic stem cells were grown successfully in culture from mice; human cultured hESCs were first described in 1998.1 The ethical issues of hESCs was one factor in the momentum that led to development of induced pluripotent stem cells, cells derived from somatic tissue, in which a combination of growth factors can induce a non-differentiated, pluripotent stem cell phenotype.2,3 All of this work occurred against a background in which the parameters of bone marrow transplants for hematopoietic disease, the first true stem cell therapy, progressed from experimental treatment to standard of care.
At its core the principle of stem cell therapy is simple: Replace a tissue or organ that is failing with the means to rebuild itself. The eye is uniquely suited for this type of therapeutic approach, as demonstrated by the long-standing success of corneal transplants. In addition to the physical accessibility of ocular structures, corneal transplant benefits from the immune privilege that reduces the risks of rejection present with other organ transplant methods. Cadaver cornea transplant isn’t appropriate for all corneal pathologies, however, and there are always patients for whom transplants simply don’t succeed, but the clinical experience that we have gained from decades of allogeneic therapies has jump-started research into stem cell-based approaches to corneal disease.
Corneal Stem Cell Therapy
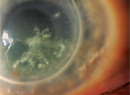
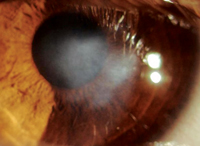
A normal cornea has its own supply of stem cells in the limbal epithelial stem cells. Localized to specialized niches at the corneal-scleral junction (the palisades of Vogt)4,5 these cells supply the entire cornea with a source of progenitor cells that ultimately replenish the corneal epithelium. Limbal stem cells respond to environmental conditions or trauma by modulating the rate of turnover and differentiation into epithelial precursors and, eventually, terminal, post-mitotic, corneal epithelium. When the health of the stem cells themselves is compromised, the ability of the cornea to repair itself is jeopardized. This limbal stem cell deficiency affects about 10 million people worldwide,6 and can result in corneal erosion, corneal vascularization and, ultimately, in visual impairment or blindness. Nearly 70 percent of corneal blindness is due to some form of LSCD.7,8
Limbal cell defects can result from either inherited or acquired conditions, and they can be partial or complete. Rare genetic disorders such as aniridia or dyskeratosis congenita are associated with LSCD, but it is more commonly the result of a trauma such as chemical exposure, fire, repeated ocular surgery involving the limbus or adverse responses to contact lens use. Diagnosis is made by clinical presentation and impression cytology.4,8,9 The presence of goblet cells on the corneal epithelium is suggestive of conjunctival epithelial ingrowth.8-10
The first step in managing LSCD is assessment and optimization of the health of the ocular surface. Surface debridement may be required, and artificial tears and topical corticosteroids may also be needed. In cases of partial and asymptomatic LSCD, this approach along with examinations may be all that is needed.8-10 When more aggressive approaches become necessary, surface debridement coupled with limbal stem cell transplantation may be necessary in order to restore the stem cell populations and reestablish a healthy, functional corneal surface.8,10
|
If the patient is not a candidate for autologous transplantation due to conditions such as bilateral LSCD, other transplant therapies may be used. A living, related donor can provide donor limbal cells or they can be harvested from otherwise healthy cadaver corneas. Both of these techniques require topical and systemic immunosuppression, and both have had only modest long-term (beyond two years) success.5,11
Patients with the most severe ocular surface disease (such as those with Stevens-Johnson syndrome) seem to have better outcomes with another approach: the combined conjunctival and keratolimbal allograft. In this procedure the patient receives tissue from a living related donor and a cadaver. This approach is useful for patients with severe ocular scarring; Stevens-Johnson patients in particular don’t respond well to traditional corneal cadaver transplants.5,6,11,12
In order to avoid the risks of immune rejection associated with allografts as well as the need for lifelong immunosuppression therapy, ex vivo expansion of autologous cells has been carried out and remains the only clinically validated stem cell-based therapy that is routinely performed in ophthalmology.6
Cultured limbal epithelial transplantation is a two-step process in which a small graft is harvested from the donor eye and then expanded in the laboratory using a tissue matrix to increase the number of progenitor corneal cells.5,12 After this ex vivo expansion, the cells are seeded onto carriers such as fibrin gels or human amniotic membrane, which can be used as a natural matrix to be transplanted into the patient.6-8 Amniotic membranes have become the most common carrier substrate used for this procedure; the membrane appears to create niche-like conditions for the donor cells, and also exhibits anti-inflammatory properties once positioned in the recipient eye. Variations on this approach of graft harvesting, cellular expansion and transplant using a donor membranous matrix show promise for LSCD; for example, seeding an expansion can be done directly on the membrane, reducing the amount of tissue needed for allografts and therefore reducing the risk of iatrogenic LSCD. This intermediate step has also improved graft success rates.5
Manufacturing Stem Cells
If autologous limbal stem cells are not available, as is the case with total LSC deficiency, another source of stem cells must be found. Alternative cell sources being investigated include: oral mucosa; hair-follicle stem cells; mesenchymal stem cells (e.g., umbilical cord lining stem cells, dental pulp stem cells and adipose tissue-derived stem cells); and embryonic stem cells.4,6-8,12-14 All of these alternative sources of stem cells have been shown to differentiate into corneal epithelium-like cells when exposed in vitro to characteristics resembling an LSC niche-like environment, but only oral mucosa cells have been evaluated clinically.12,13
Cultivated oral mucosal epithelial transplantation uses autologous cells obtained from the inferior buccal mucosa. Short-term results have been positive, but long-term efficacy has yet to be assessed.12,13 Most commonly described methods include ex vivo expansion of excised mucosal tissue, with culture conditions designed to select for epithelial progenitor phenotypes. Cell expansion occurs in culture plates or using an amniotic membrane. Success with the ex vivo LSC expansion means that regardless of the autologous cell donor tissue, some variation on the ex vivo expansion paradigm is likely to be part of treatment protocols going forward. The exception to this will be protocols employing embryonic or induced pluripotent donor cells.
Human embryonic stem cells are derived from the inner cell mass of a 3- to 5-day-old embryo. Embryonic stem cells are more robust than adult somatic stem cells and they have a theoretically unlimited capacity to divide, and are by definition pluripotent: They can differentiate into all cell and tissue types given the correct sequence of differentiating stimuli. The establishment of hESC lines, as well as the ability to obtain single-cell biopsies without harming embryos has eliminated some of the religious and ethical concerns that surrounded earlier stem cell research.14 Objections exist, for example, over the use of established hESC lines in research.
The task of promoting appropriate differentiation has been addressed by growing hESCs on a collagen matrix using a nutrient broth similar to what is found in vivo in the limbal stem cell niche. When these cells are transplanted onto human corneas in vitro, they behave according to the normal corneal epithelial developmental process, forming corneal epithelial-like cells.15
While these studies provide useful insights into how hESCs may be used for corneal disease, the use of embryonic cells may eventually give way to cells derived from the reprogramming or inducing of non-embryonic stem cells into becoming pluripotent, undifferentiated stem cells. Induced pluripotent stem cells are adult somatic stem cells that have been reprogramed to return to an embryonic state, and so would provide tissue necessary for allografts of any tissue type.16
The reprogramming has been possible using combinations of transcription factor cocktails; most of these mixes include transcription factors Oct4 and Sox2 in combination with several other factors. Somatic cells engineered to express these mixes regress to an undifferentiated state; stable expression maintains this iPSC state, which can then be directed toward a new path of differentiation. Several reports have described a method that successfully produced corneal epithelial cells from iPSCs.17,18 In one of these studies, mature human dermal epithelial cells were harvested from a donor and reprogrammed into iPSCs by a cocktail of transcription factors including Oct3/4, Sox2, c-Myc and Klf4. They were then differentiated into corneal epithelial cells using a stromal cell-derived inducing activity. Immunofluorescent staining of corneal epithelial markers was absent at the iPSC stage, but returned when cells were induced to differentiate.18 This proof-of-concept study demonstrates the potential for iPSCs to provide the tissue needed for an inexhaustible supply of transplantable cells.
What Cost Pluripotency?
Although researchers have demonstrated that reprogramming somatic cells to pluripotency is possible, significant challenges remain. Some of the factors used to induce the iPSC state are associated with oncogenic transformation (such as c-Myc), and the risk of teratoma formation needs to be evaluated. The ability of iPSC to differentiate into cells that are able to work in concert with existing cells also needs to be determined.16 It turns out that getting iPSCs to successfully differentiate has been more difficult than expected. A promising method that may help in predicting (and therefore, achieving) success involves the expression of a specific cell marker, p63, in the iPSCs.5,19 Several studies established that the likelihood of transplant success is positively correlated with the number of p63-positive cells in the culture. This observation may lead to an ability to predict iPSC transplant success,6 and has been an important factor in the early efforts to commercialize corneal stem cell therapies.20
The use of animal products in cell culturing, such as mouse feeder cells and fetal calf serum, is another area of concern. The risks of xeno-contamination and zoonotic disease transmission are unknown, so animal-product-free systems are being explored. Similar efforts are being applied to the ex vivo expansion techniques described earlier. To circumvent these issues, one study employed autologous limbal stem cells grown in the patient’s own serum on an FDA-approved contact lens instead of using mouse feeder cells and human amniotic membranes. Early results are promising.
The clinical experience with corneal transplants and the availability of donor tissue have given anterior segment stem cell research a sizeable head start on posterior segment efforts, but a strong focus on stem cell therapies for congenital retinal diseases and on conditions such as dry AMD will soon level the playing field. As an example, a Phase I/II study of hESC therapy for macular degeneration was recently published, with promising safety and graft survival data providing an encouraging outlook for the future.21 Look for a discussion of stem cell therapeutics beyond the cornea in a future column.
Finally, it’s important to recognize that, like all new therapeutic strategies, the viability of these promising technologies depends upon the ability to refine the techniques to the point where they can be economically viable. Efforts to address this issue are focusing on the technologies required to create a consistent supply of high- quality iPSCs that are safe and respond to reprogramming in a reliable, predictable way.22 We have no doubt that the last remaining hurdle can be cleared, however, and that more clinical trials and approved indications for ocular stem cell therapies will be part of our future. REVIEW
Dr. Abelson is a clinical professor of ophthalmology at Harvard Medical School. Dr. McLaughlin is a medical writer at Ora Inc.
1. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998;282:1145-1147.
2. Junying Yu J, Vodyanik MA, Smuga-Otto K, et al. Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007;318:1917-1920.
3. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;30:131:5:861.
4. Dhamodaran K, Subramani M, Ponnalagu M, Shetty R, Das D. Ocular stem cells: A status update. Stem Cell Research & Therapy 2014;5:56.
5. Menzel-Severing J, Kruse FE, Schlötzer-Schrehardt U. Stem cell-based therapy for corneal epithelial reconstruction: Present and future. Can J Ophthalmol 2013;48:1:13-21.
6. Menzel-Severing J. Emerging techniques to treat limbal epithelial stem cell deficiency. Discov Med 2011;11:56:57-64.
7. Sun TT, Lavker RM. Corneal epithelial stem cells: Past, present, and future. J Investig Dermatol Symp Proc 2004;9:3:202-7.
8.Ahmad S. Concise review: Limbal stem cell deficiency, dysfunction, and distress. Stem Cells Transl Med 2012;1:2:110-5.
9. Watts FM Driskell RR. The therapeutic potential of stem cells. Phil. Trans R Soc B 2010;365:155–163.
10. O’Callaghan AR, Daniels JT. Concise Review: Limbal epithelial stem cell therapy: Controversies and challenges. Stem Cells 2011;29:12:1923-32.
11. Marchetti V, Krohne TU, Friedlander DF, et al. Stemming vision loss with stem cells. J Clin Invest 2010;120:9:3012–3021.
12. Hirayama M, Satake Y, Higa K, et al Transplantation of cultivated oral mucosal epithelium prepared in fibrin-coated culture dishes. Invest Ophthalmol Vis Sci 2012;21:53:3:1602-9.
13. Ma DH, Kuo MT, Tsai YJ, et al. Transplantation of cultivated oral mucosal epithelial cells for severe corneal burn. Eye 2009;23:1442-50.
14. Chung Y, Klimanskaya I, Becker S, Li T, Maserati M, Lu SJ, Zdravkovic T, Ilic D, Genbacev O, Fisher S, Krtolica A, Lanza R. Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell 2008;7:2:2:113-7.
15. Hanson C, Hardarson T, Ellerström C, Nordberg M, Caisander G, Rao M, Hyllner J, Stenevi U. Transplantation of human embryonic stem cells onto a partially wounded human cornea in vitro. Acta Ophthalmol 2013;91:2:127–130.
16. Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol 2011;13:5:497-505.
17. Mikhailova A, Ilmarinen T, Uusitalo H, et al. Small-molecule induction promotes corneal epithelial cell differentiation from human induced pluripotent stem cells. Stem Cell Reports 2014;6:2:219-31.
18. Hayashi R1, Ishikawa Y, Ito M, Kageyama T, Takashiba K, Fujioka T, Tsujikawa M, Miyoshi H, Yamato M, Nakamura Y, Nishida K. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. PLoS One 2012;7:9:e45435.
19. Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 2010;363:147-55.
20.http://www.nature.com/news/behind-the-scenes-of-the-world-s-first-commercial-stem-cell-therapy-1.17022
21. Schwartz SD, Regillo CD, Lam BL et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase I/II studies. Lancet 2015;385:509-516.
22. Marli Silva, Laurence Daheron, Hannah Hurley, et al. Generating iPSCs: Translating cell reprogramming science into scalable and robust biomanufacturing strategies. Cell Stem Cell 2015;16:13-17.