Ironically, many topical drops we prescribe to treat glaucoma work by reducing inflow; often, aqueous suppressants are used as initial or additive therapy. One might argue that this is not equivalent to ciliary ablation because medication effects are reversible. However, it’s worth remembering that while cyclophoto-coagulation is not reversible, it is titratable; we only ablate a portion of the ciliary processes. (Besides, even treating 360 degrees won’t completely stop aqueous production.)
Recently, endoscopic cyclophoto-coagulation has come to the forefront because it’s currently often done in combination with cataract surgery, making it an adjunctive procedure in the early stages of glaucoma. Because it is a minimally invasive glaucoma surgery, one could also put it into the category of MIGS.
I should make it clear that I don’t perform a great deal of ciliary ablation. However, endoscopic cyclo-photocoagulation (and transscleral cyclophotocoagulation) are part of our armamentarium, and I think we should have as many tools as possible at our disposal. There are times when one of these procedures may be the right procedure to help your patient.
A Brief History
In the past, surgeons have tried to limit ciliary production of aqueous using surgical excision, penetrating diathermy (i.e., cautery), cryotherapy, ultrasound and lasers of different kinds. Transscleral cyclophotocoagu-lation is now most commonly per-formed using a semiconductor diode G-probe that contacts the eye. The laser energy is absorbed by the pig-mented ciliary epithelial tissues, re-sulting in destruction of the cells.
It was Martin Uram, MD, who first designed and developed the endoscopic laser in Little Silver, N.J. (current home of Endo Optiks, manufacturer of the instrument). It uses an 810-µm diode continuous- wave laser that allows you to “paint” the ciliary processes. The reusable probes, available in curved or straight versions and in different sizes, contain a light source, video camera and the laser, with an aiming beam that allows you to see the area you’re treating. The optics of the system provide enough depth of focus—from 1 to 30 mm—to let you pull back and get a wider field of view when necessary. As a retina specialist, Dr. Uram used the instrument through the pars plana to treat the ciliary processes when he was doing vitrectomies. Later it was converted for use through the limbus or through a corneal wound during cataract surgery. Today, ECP is usually done through a clear cornea cataract surgery incision. (ECP can also be done as a stand-alone procedure in pseudophakic patients.)
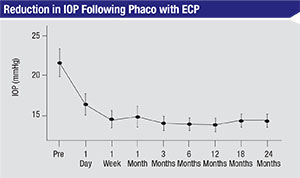 |
| A retrospective review of 56 phaco-ECP patients found that IOP was reduced from a mean of 21.5 mmHg at baseline to 14.4 mmHg at 18 and 24 months (p<0.01). Medications were reduced from a mean of 2.1 at baseline to 2.0 at 24 months (p>0.05).3 |
Performing the Procedure
When I perform ECP in the OR in conjunction with cataract surgery, I use topical lidocaine gel and then inject a small amount of lidocaine intracamerally at the time of the pro-cedure. (As a general rule, I don’t do retrobulbar injections for any surgical procedures.) When I do TCP in the treatment room (i.e., not in conjunction with cataract surgery), I always use a sub-Tenon’s injection of lidocaine 2%/marcaine 0.375% for anesthesia.
When done in conjunction with cataract surgery, ECP is performed after the lens implant is in place. I work through the cataract wound (usually a clear-cornea, limbal, 2.5-mm incision); I place viscoelastic into the sulcus, which elevates the iris and lowers the IOL, providing a direct line of sight to the ciliary process. A pars plana approach may be undertaken by vitreoretinal surgeons following a pars plana vitrectomy.
It’s important to learn to watch the monitor and occasionally glance at the eye during the procedure, to make sure the anterior chamber is acceptable, without significant torque of the eye. During the treatment, as you “paint” the ciliary process, you’re looking for blanching and shrinking of the process; you don’t want to see popping or explosions. This is one of the differences between ECP and TCP; I once filmed a transscleral treatment using the endoscope, and you could see some of the ciliary pro-cesses exploding as they absorbed the laser energy.
Histology of eyes that have un-dergone TCP shows widespread tissue disruption, signs of a pro-longed reduction in blood flow and coagulative necrosis of the ciliary stroma. In contrast, during ECP you see blanching and shrinking but no exploding tissue. Histopathology after ECP demonstrates localized contraction of the ciliary processes, signs of a temporary reduction in iris root/ciliary process perfusion, less architectural disorganization and sparing of the ciliary muscle. (The impact of the last factor may be muted by the fact that the accommodation is no longer an issue after standard cataract surgery.) It’s not clear whe-ther one approach or the other leads to a greater reduction in pressure.
As noted, this treatment is titrat-able. (Malik Kahook, MD, at the University of Colorado, Denver, did a retrospective, consecutive case re-view of 40 patients who underwent combined ECP and phaco, and found greater reduction in IOP at both three and six months when 360 degrees were treated, compared to when less than 300 degrees were treated.1) Using the curved probe, I’m able to treat 270 degrees through one incision; if you want to treat 360 degrees, you’ll need to make a second incision. Once you’re finished, it’s important to remove the viscoelastic, because viscoelastic that’s left behind is a significant cause of pressure spikes after the procedure.
Postoperatively, I inject a miotic such as acetylcholine or carbachol, along with topical steroids and anti-biotics, just as I would following any cataract surgery. I keep the patient on pressure-lowering medications until the pressure comes down, but I continue steroids a bit longer than I would after standard cataract surgery. (After cataract surgery I start to taper the steroid drops after a week. Following cataract surgery with ECP, I may keep the patient on the steroids for three or four weeks, tapering the patient off by six weeks.) Non-steroidal topical agents may also be used in some patients.
“ECP plus” is another variation of the procedure, done through the pars plana incision, a variation most likely to be performed by a retina specialist. The “plus” refers to additionally treat-ing part of the pars plana. The ciliary epithelium not only covers the ciliary process—the structure that comes out from the wall of the eye—but also the pars plana, which can be thought of as a very anterior part of the retina. Since that area does have ciliary epithelial cells, you can treat the anterior 1 to 2 mm of the pars plana to generate additional reduction in aqueous. Usually, this would be done as part of a standard pars plana vitrectomy, with the probe inserted through the sclerostomy.
Indications
Today, the most common use for ECP is as primary therapy combined with phaco. However, it is also used in a number of other situations:
• combined with other MIGS procedures (as in the ICE procedure, consisting of iStent, cataract extraction and ECP);
• to treat refractory glaucoma;
• if TCP fails to lower pressure (direct visualization via ECP may yield an improved outcome);
• as concomitant therapy with pars plana vitrectomy;
• to treat aphakic and pseudophakic pediatric glaucoma;
• in patients with a history of scleral disease. For example, some pa-
tients have a very thin sclera, (e.g., osteogenesis imperfecta). In these cases, you don’t want to do an inva-sive incisional procedure such as a trabeculectomy or a tube shunt.
• to address plateau-iris syndrome, in which the eye has an anterior rotation of the ciliary process, by performing endocycloplasty. Cyclo-ablation causes shrinkage of the ciliary process, rotating it backwards, which helps to open up the peripheral angle.
• for endoscopic visualization. Using the probe can be very help-ful if you have a displaced or im-properly placed implant; you can see precisely where the IOL haptics are and whether they’re in the wrong position. Similarly, the endoscopic system is helpful when patients have corneal disorders that obstruct the surgeon’s view inside the eye; surgical procedures can still be done, using the endoscopic probe to see what’s going on behind the corneal or lenticular opacity. (The endoscope can also be used as a diagnostic tool in these circumstances.)
Caveats and Complications
Several things should be considered relative contraindications for ECP:
• Pseudoexfoliation glaucoma. Because this disease leaves a white, fibrillar material on the ciliary pro-cesses, they may not absorb the laser energy as well.
• Weakened zonules. It’s important to be especially careful if you perform ECP in patients with weakened zonules. The probe is very close to the lens during the procedure, and over the course of the procedure the viscoelastic that’s maintaining the space between the lens implant and the iris gradually diffuses into the anterior chamber, causing the opening in which you’re working to become shallower. As that happens, it becomes possible for the probe to touch the lens implant. That’s not a problem, as long as you don’t put any pressure on the lens; but if for some reason you do—I’ve seen trainees make this mistake—you may stretch or break some of the zonules.
• A history of inflammatory eye disease or cystoid macular edema. ECP patients will have more postoperative inflammation than patients who undergo standard cata-ract surgery. CME is a possible com-plication, making patients with this kind of history more likely to have trouble postoperatively.
Although ECP is generally a safe procedure, complications can occur. These include:
• Inflammation.
• Hyphema. (This has been re-ported, although I’ve never seen it during any of my procedures.)
• Cystoid macular edema. As noted above, this can occur, especially in those who have a history of CME.
• Zonular damage. This can occur if the surgeon accidentally pushes on the lens during the procedure.
• Hypotony or phthisis. In theory, this could happen after the procedure, but I’m not sure it’s ever been reported.
• Postoperative change in refractive error. This could theoretic-ally happen as a consequence of a change in effective lens position caused by inadvertent pressure placed on the lens implant. One study looked at postoperative refractive error changes following ECP; the data revealed some slight differences, but they were not significant.2
What the Data Show
How effective is treatment with ECP when combined with cataract surgery? Here are the data from a number of studies:
| Allergan Displays ForSight |
| In August, Allergan announced its intent to purchase eye-care company ForSight Vision5, which has a sustained-release drug device in development. Per the agreement, Allergan will acquire ForSight Vision5 for an initial $95 million payment and a launch milestone payment related to the company’s lead product, the sustained-release device. ForSight’s device is a periy-ocular ring that’s preservative-free and rests on the surface of the eye beneath the lid. In its current iteration, it releases the glaucoma drug bimatoprost over a period of months to help lower intraocular pressure while avoiding issues with patients’ adherence to glaucoma drop schedules. “We know if we’re going to take care of thousands of patients, we need a lot of tools in our bag,” says Jay Parekh, MD, vice president for medical affairs and global lead for eye care at Allergan. “And, when you have a disease therapy that could run a 20- or 30-year course, compliance is a key aspect of it.” —The Editors |
• A retrospective review of 56 phaco-ECP patients found that IOP was reduced from a mean of 21.5 mmHg at baseline to 14.4 mmHg at 18 and 24 months (p<0.01). Medications were reduced from a mean of 2.1 at baseline to 2.0 at 24 months (p>0.05).3
• A retrospective review of 63 phaco-ECP eyes that received 270 to 360 degrees of treatment looked at the 12-month follow-up.4 Mean IOP dropped from 21.1 ±6.2 at baseline to 16.1 ±5.27 mmHg (p<0.01). Medications dropped from 2.7 ±1.1 to 1.47 ±1.3 (p<0.01). (How much pressure lowering can be attributed to the phaco alone is difficult to say, be-cause there was no control group.) Of note, the outcomes were best in older patients and those with a higher baseline IOP. Whether this reflects a difference in the vascularity of the ciliary cells, or a different response to a change in inflow due to age or high pressure, is impossible to determine.
Notably, all medications in this group were discontinued after surgery and re-introduced as needed. I suspect this may be the reason they found more of a decrease in the number of medications than many studies find. In general, we discontinue all medications after a full-fledged glaucoma surgery, but many times with MIGS procedures, especially if the patient has moderate disease, we’re more hesitant to do so. As a result, we may be not seeing the full effect of these treatments, at least in terms of their ability to decrease medication use.
This study also looked at complications. The data showed a small amount of induced astigmatism (possibly related to movement of the lens); posterior vitreous detachment, a few cases of increased IOP, and an unusually high 11 percent of cases having fibrinous uveitis, an event I’ve rarely seen following ECP. Overall, the study authors concluded that the procedure was safe and effective, with a success rate—defined as a greater-than-20-percent reduction in IOP—of 55.5 percent.
• A prospective, nonrandomized, matched-control study compared phaco with ECP to phaco alone in 160 patients (80 per group) matched for age and baseline IOP.5 In the study group, mean IOP decreased from 18.1 ±3 mmHg to 16.0 ±3.3 at two years, with medication use dropping from 1.5 ±0.8 to 0.4 ±0.7. The phaco-only group decreased from 18.1 ±3 mmHg to 17.3 ±3.2 at two years, with medications decreasing from 2.4 ±1.0 to 2.0 ±1.0. The difference in pressure and medication reduction between the groups was statistically significant at all time points.
• Another retrospective chart review with a 36-month follow-up compared phaco with ECP to phaco alone; 261 eyes underwent combined surgery, while 52 eyes underwent only cataract surgery.6 The difference between the IOP-lowering in the two groups wasn’t significant at 16 weeks (14.6 mmHg vs. 15.5 mmHg, p=0.34). However, the percentage of patients meeting the definition of full success (a greater-than-20-percent reduction in IOP and a decrease of one medication) was significantly different between the two groups: 61.4 percent vs. 23.3 percent (p<0.001). Likewise, comparing the percentage in each group that achieved qualified success (IOP lower than baseline with a decrease of one medication), the difference between the groups was also significant: 72.6 percent vs. 23.3 percent (p<0.001). Put another way, those who underwent cataract surgery alone had a one-in-four chance of getting a significant pressure reduction and a decrease in medications. Those who underwent the combination surgery had three times better odds of this result.
• Finally, one retrospective chart review looked at the combined procedure in 104 eyes of 104 patients with advanced glaucoma at the time of surgery.7 Follow-up was 17.3 ±1.8 months. Among these patients, mean IOP decreased from 17 ±1.4 mmHg to 14.7 ±1.3 mmHg. Only 11.9 percent achieved absolute success, defined as an IOP ≤15 mmHg on no medications; however, 72.3 percent achieved qualified success, defined as an IOP ≤15 mmHg with medications.
Because ECP—often combined with phaco—can be considered a MIGS procedure, it would be helpful to have some prospective, randomized studies comparing it to the other MIGS procedures. So far, I’m not aware of any studies fitting that description. However, we presented a poster last year at both the American Academy of Ophthalmology and American Glaucoma Society annual meetings showing data from a re-trospective study of 150 patients at the Barnes-Jewish Hospital/Washington University School of Medicine; 41 underwent phaco alone; 52 un-derwent phaco with ECP; and 57 underwent phaco with Trabectome. As expected, higher IOPs, more medications and worse disease had influenced the surgeons to choose a combined procedure.
Among the results:
• There was significant pressure lowering with both phaco-Trabectome and phaco-ECP, as well as higher rates of success (defined as a reduction in IOP of at least 20 percent, or a reduction of at least one medication) compared to phaco alone.
• Success rates of the two combined procedures weren’t significantly dif-ferent at 24 months, although there was a trend toward better pressure reduction with phaco-Trabectome.
• In terms of complications, there was more hyphema with phaco-Trabectome and more CME with phaco-ECP.
It’s worth noting that because the study was retrospective, patients were not matched for preoperative IOP, medications or disease severity.
Increasing Popularity
There’s no question that the use of ECP has expanded significantly over the past 10 years. Starting in 2005, the single billing code for ciliary ablation was separated into transscleral procedures and endo-scopic procedures. By 2012, use of transscleral procedures dropped from 5,978 to 3,268, a 45-percent decrease. During the same period, ECP procedures grew from 5,383 to 10,728, a 99-percent increase. Of note, reimbursement for ECP is significantly greater than for trans-scleral procedures—$635.84 vs. $434.57; ECP is a more involved pro-cedure performed in the OR.
Given that ECP has a good track record for safety, and may produce significant IOP lowering following cataract surgery—with minimal risk—it’s an adjunctive treatment worth considering. REVIEW
Dr. Siegfried is the Jacquelyn E. and Allan E. Kolker, MD, Distinguished Professor of Ophthalmology at Wash-ington University School of Medicine in St. Louis. She has no financial in-terest in any product discussed.
1. Kahook MY, Lathrop KL, Noecker RJ. One-site versus two-site endoscopic cyclophotocoagulation. J Glaucoma 2007;16:6:527-30.
2. Sheybani A, Saboori M, Kim JM, Gammon H, Lee AY, Bhorade AM. Effect of endoscopic cyclophotocoagulation on refractive outcomes when combined with cataract surgery. Can J Ophthalmol 2015;50:3:197-201.
3. Lindfield D, Ritchie RW, Griffiths MF. ‘Phaco-ECP’: Combined endoscopic cyclophotocoagulation and cataract surgery to augment medical control of glaucoma. BMJ Open 2012;2:3.
4. Clement CI, Kampougeris G, Ahmed F, Cordeiro MF, Bloom PA. Combining phacoemulsification with endoscopic cyclophotocoagulation to manage cataract and glaucoma. Clin Experiment Ophthalmol 2013;41:6:546-51.
5. Francis BA, Berke SJ, Dustin L, Noecker R. Endoscopic cyclophotocoagulation combined with phacoemulsification versus phacoemulsification alone in medically controlled glaucoma. J Cataract Refract Surg 2014;40:8:1313-21.
6. Siegel MJ, Boling WS, Faridi OS, Gupta CK, Kim C, Boling RC, Citron ME, Siegel MJ, Siegel LI. Combined endoscopic cyclophotocoagulation and phacoemulsification versus phacoemulsification alone in the treatment of mild to moderate glaucoma. Clin Experiment Ophthalmol 2015;43:6:531-9.
7. Morales J, Al Qahtani M, Khandekar R, Al Shahwan S, Al Odhayb S, Al Mobarak F, Edward DP. Intraocular Pressure Following Phacoemulsification and Endoscopic Cyclophotocoagulation for Advanced Glaucoma: 1-Year Outcomes. J Glaucoma 2015;24:6:e157-62.



