Prostaglandin analogues are the first-line choice for addressing elevated intraocular pressure in a glaucoma patient or suspect (provided that there are no contraindications or preexisting allergies to any of the components). Given the once-a-day dosing, significant IOP reduction and very low systemic side-effect profile that we usually associate with a prostaglandin, this makes sense. However, some patients appear to have a positive response that’s maintained for some time after the drug is discontinued—something we don’t see with other pressure-lowering medications. Here, I’d like to share some of the evidence that this really does happen in some patients, and offer some possible explanations for it.
Is This Phenomenon Real?
Many doctors find it hard to accept the idea that a medication might have an effect even after it’s discontinued. I’m sure that’s true in part because it doesn’t happen in every patient. Furthermore, the way data from some clinical trials is reported may obscure the phenomenon, because outcomes from all patients are averaged together. However, there is data that supports the existence of this phenomenon—and many physicians managing glaucoma (including me) have also seen it first-hand.
Consider the data from one Allergan-sponsored study, headed by Randy Craven, MD.1 This Phase I/II, prospective, 24-month, dose-ranging, paired-eye controlled clinical trial evaluated the efficacy and safety of the bimatoprost SR sustained-release implant (Durysta, Allergan) over a 24-month period, compared to topical bimatoprost 0.03% applied daily in the fellow eye. An unexpected finding was that some patients showed a sustained IOP reduction in the trial eye, long after the bimatoprost implant had dissolved. At one year, 40 percent of eyes still had an IOP significantly lower than baseline; at two years, 28 percent still exhibited this phenomenon. (The implant typically dissolves in three to four months.)
I personally saw this happen a couple of years ago when I treated a patient I’d diagnosed with primary open-angle glaucoma. His pre-treatment pressure was 24 mmHg; I was able to get his pressure down to 14 mmHg using topical medications, including bimatoprost, without needing any surgery. Several years later, we enrolled that patient in a clinical trial and washed him out. To our surprise, he failed to meet the entry criteria for the trial because his pressure only went up to 19 mmHg after the washout—not back to his original pressure of 24 mmHg. We were left scratching our heads and wondering what happened.
Others have observed this as well. A study headed by Henri Jampel, MD, looked at IOP in 1,400 eyes in the HORIZON and COMPASS trials.2 These patients had been managing their IOP with up to four medications prior to the trials; even before washout, a fair number were still above 21 mmHg. But after the washout for these studies, many of them remained below 21 mmHg (see graph below).
An even more scientific study of this phenomenon is a prospective, randomized, controlled study conducted by Cynthia Hutnik, MD, and her group in Toronto.3 They took a group of patients that were using PGA monotherapy—a single prostaglandin. These patients were confirmed to have high IOPs at baseline—between 21 and 38 mmHg. They’d been on topical PGA therapy for more than six months. The patients were randomized into two groups: One group was washed out for up to six weeks, while the control group was not.
The control group’s mean IOP didn’t change at all, and as expected, the washed-out group’s mean IOP went up after the prostaglandin monotherapy was stopped. However, the mean doesn’t tell the whole story; if you look at the scatter-plot (see second graph below), a large number of patients actually kept their IOP below 21 after washout. So, it seems clear that the treatment caused some sort of permanent—or at least semi-permanent—change. We know it lasted at least longer than six weeks. And, this was seen after using topical prostaglandin drops, not a sustained-release implant.
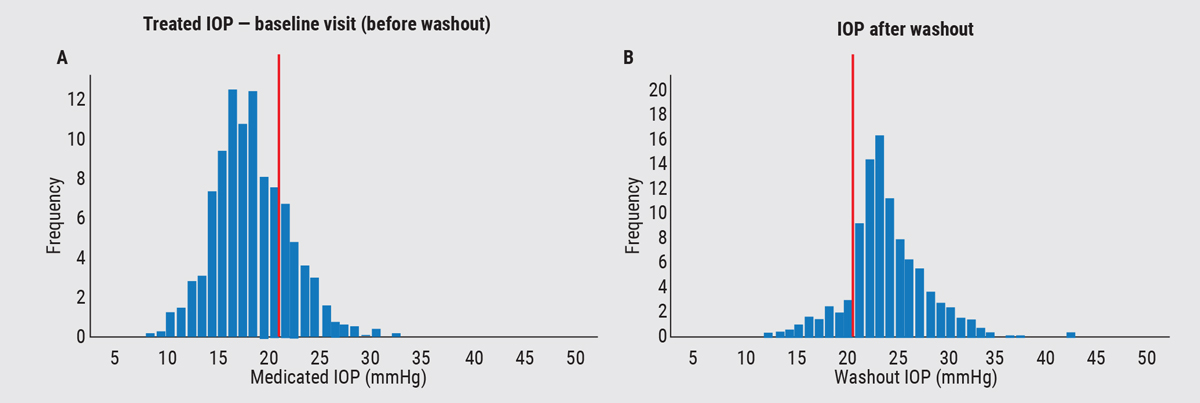 |
| A 2020 study by Johnson and Jampel2 looked at 1,400 eyes that were supposed to enroll in the HORIZON and COMPASS studies (for the Hydrus and CyPass devices). Patients were on 0 to 4 medications before washout. After washout, many eyes remained below 21 mmHg. |
Mechanisms We Know About
All of this raises the question: What exactly are prostaglandins doing that might account for this? One mechanism has been well-established. Work from multiple studies, nicely summarized in a review article from Duke’s Daniel Stamer, PhD, and colleagues4 that includes some work done by our group, has shown that prostaglandins change the ratio of matrix metalloproteinases (MMPs) to tissue inhibitors of matrix metalloproteinases (TIMPs) in the trabecular meshwork and ciliary body, as well as in scleral fibroblasts.
MMPs are enzymes that break down extracellular matrix, thus allowing enhanced aqueous outflow. TIMPs are kinetic inhibitors of MMPs; they block the active sites on the relevant molecules and thus prevent them from downgrading the matrix. So, the extent of extracellular matrix degradation—and increased outflow—is determined by the ratio of MMPs to TIMPs. (In fact, an imbalance in the MMP/TIMP ratio may be involved in the elevated IOP often associated with glaucoma.) By changing that ratio in favor of MMPs, prostaglandins allow more breakdown of the matrix and greater outflow, thus lowering IOP.
Normally, the ratio of MMPs to TIMPs causes some outflow through the trabecular meshwork. But what some of our group’s work has shown is that although prostaglandins alter the amount of extracellular matrix breakdown in both the trabecular meshwork and the ciliary body, the amount of change is greater in the ciliary body.5,6 Because prostaglandins seem to affect the ciliary body more than the trabecular meshwork, the ciliary body becomes more permeable, and the amount of outflow through the ciliary body ends up becoming greater than the outflow through the trabecular meshwork.
All these assertions are well-established by about 35 years of research.
Using a sustained-release device to dispense bimatoprost adds some complexities to this equation. First, it’s been demonstrated that when the bimatoprost SR implant is in place, it achieves high target tissue concentrations.7 Our group and others have shown that the effect of bimatoprost is dose-dependent.5,6,8,9 In essence, the more prostaglandin you give, the more effect on the MMPs you get. Another change when using sustained release is that the patient doesn’t get the pulsed dosing provided by topical drops; instead the patient gets continuous dosing. That may confer some advantage and/or an additional mechanism of action.
It’s reasonable that increased porosity of the matrix tissue could remain after the change in the ratio of MMPs to TIMPs has returned to baseline. Yes, we’re talking about living tissue that changes and may regenerate, but that regeneration could take some time to occur.
What Else Might Be Going On?
Despite these advances in our understanding of prostaglandins, there’s plenty more that remains to be discovered. Questions include: Why is this effect more noticeable with the bimatoprost implant than with topical prostaglandin drops? And, why is this lingering effect only seen in some patients, not all?
Some answers to these questions have been suggested by Dr. Stamer in an abstract from the 2021 Association for Research in Vision and Ophthalmology meeting.10 (We participated in some of this work, but Dr. Stamer was the primary investigator. In the interest of disclosure, Dr. Stamer has received research funding from Allergan.) His work showed that:
- Bimatoprost and bimatoprost free acid (BFA)—the latter being the metabolized version of bimatoprost that impacts tissues inside the eye when only topical drops are used—have different effects on MMP gene expression in cells cultured from human outflow tissues (i.e., trabecular meshwork, ciliary body and scleral cells). Levels of bimatoprost seen with an implant caused a dramatic upregulation of MMP-1 in trabecular meshwork cells and ciliary cells, compared to the impact of the levels of BFA seen with topical drops.
- Bimatoprost and BFA had different effects on MMP gene expression. The implant’s high levels of bimatoprost altered the expression of 11 partially overlapping genes in trabecular meshwork and ciliary muscle cells, including a dramatic upregulation in MMP-1 in trabecular meshwork cells. Typical levels of BFA seen with the use of topical drops only significantly altered the expression of two genes.
- There were noticeable differences in the response of different cell strains from different individuals. This may partly explain the differences in individual long-term responses to prostaglandins—i.e., why some people get the sustained effect and others don’t.
- A key difference was seen in the MMP-1 expression changes found in glaucomatous cells vs. normal cells. Implant levels of bimatoprost didn’t affect most genes differently in glaucomatous vs. normal cells, but the expression of MMP-1 and MMP-10 increased dramatically in glaucomatous cells compared to normal cells.
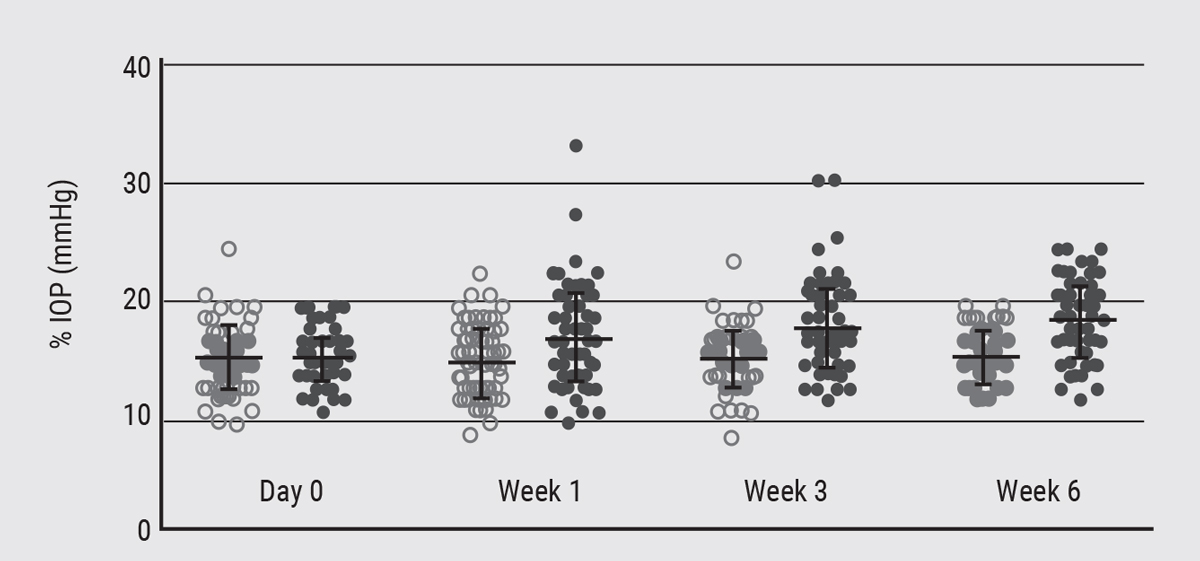 |
| Another 2020 study3 looked at patients who had been on PGA monotherapy for more than six months. Before treatment, IOPs ranged from 21 to 38 mmHg. After washout, mean IOP for these eyes did go up, but the majority continued to have an IOP below 21 mmHg. |
The Lymphatic Factor
There’s another possible explanation for the IOP-lowering effect of prostaglandins, which we’re now investigating. Some time ago, I attended a presentation given by Elke Lütjen-Drecoll, MD, at the annual ARVO meeting.11 She’d given topical bimatoprost to monkeys for one year. On histology, she observed spaces in the ciliary muscle tissue, particularly surrounding large blood vessels. When she went to higher magnification using electron microscopy, she found that these spaces were long, straight running tubes, incompletely lined with endothelial-like cells, making them likely to persist.
I remember her asking the audience—a group of established trabecular meshwork and outflow molecular biologists—what they thought about this. There was dead silence in the room. I was stunned that these experts didn’t know what to make of this, and her observation stuck with me.
What she observed sounded to me very much like lymphatics. While this isn’t widely known, at least two papers have shown the presence of lymphatics in ciliary body tissue.12,13 Prior to 2000, it wasn’t known that lymphatics existed in the eye, so these papers broke new ground.
In any case, the possibility that such a phenomenon would be connected to improved aqueous outflow isn’t hard to imagine. Thus, our group’s current hypothesis is that the bimatoprost free acid induces lymphangiogenesis, or something akin to it, creating the endothelial-lined passages observed by Dr. Lütjen-Drecoll. Furthermore, we suspect that PGAs induce lymphatic vessel formation, as opposed to simply increasing flow through existing lymphatics. (Of course, if true, this would be in addition to factors such as the altered ratio of MMPs and TIMPs, and the gene expression uncovered in Dr. Stamer’s work.)
Our research in this area is still in its early stages. We’re looking for protein markers associated with lymphangiogenesis following the extended use of prostaglandins, and so far, in a very small sample of two eyes, we have indeed found them. Our preliminary data also suggests that there may be a dose-response effect for some of these proteins. In addition, we’re dosing mice to see if we can identify a sub-population that exhibits this prolonged effect. If histological findings confirm what we’ve seen in human cells, that would convincingly support the lymphatic hypothesis.
Again, this work is in its early stages, so it’s impossible to predict what the ultimate outcome of the study will be.
A Work in Progress
So: What do we know so far?
- After the use of prostaglandins, prolonged IOP reduction is seen in some patients, even after washout.
- PGAs cause a dose-dependent response that induces a change in the ratio of MMPs and TIMPs in trabecular meshwork tissue, ciliary body smooth muscle cells and scleral fibroblasts, favoring extracellular matrix turnover. This leads to enhanced uveoscleral and conventional outflow (favoring the uveoscleral pathway).
- Prostaglandins’ effect on gene expression of MMPs is variable in different individuals. This may explain the long-term IOP-lowering effect seen in some patients but not others. Expression of MMP-1 may be responsible.
- Although this remains unproven at this point, we believe that bimatoprost induces lymphangiogenesis in some patients, contributing to the persistent IOP-lowering effect seen in those individuals.
Now that it’s clear that some patients will experience an ongoing pressure reduction after cessation of a prostaglandin, an obvious unmet need is to be able to identify which patients will have this sustained response. In the meantime, as clinicians, we certainly don’t want to tell patients not to take their drops because there’s a chance they might continue to work.
I believe it’s fair to say that this ongoing work reinforces our belief that prostaglandin analogues are an excellent first-line topical agent (provided that there are no contraindications or preexisting allergies to any of the components), with the potential for residual effect. That effect merits further study, especially with regard to the sustained-release bimatoprost implant.
This article has no commercial sponsorship.
Dr. Singh is a professor of ophthalmology and chief of the Glaucoma Division at Stanford University School of Medicine. Dr. Netland is Vernah Scott Moyston Professor and Chair at the University of Virginia in Charlottesville.
1. Craven ER, Walters T, Christie WC, et al. 24-month Phase I/II clinical trial of bimatoprost sustained-release implant (bimatoprost SR) in glaucoma patients. Drugs 2020;80:2:167-179.
2. Johnson TV, Jampel H. Intraocular pressure following prerandomization glaucoma medication washout in the HORIZON and COMPASS trials. Am J Ophthalmol. 2020;216:110-120
3. Lim CW, Diaconita V, Liu E, et al. Effect of 6-week washout period on intraocular pressure following chronic prostaglandin analogue treatment: a randomized controlled trial. Can J Ophthalmol 2020;55:2:143-151.
4. Weinreb RN, Robinson MR, Dibas M, Stamer WD. Matrix metalloproteinases and glaucoma treatment. J Ocul Pharmacol Ther 2020;36:4:208-228.
5. Oh DJ, Martin JL, Williams AJ, et al. Analysis of expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human ciliary body after latanoprost. Invest Ophthalmol Vis Sci 2006;47:3:953-63.
6. Oh DJ, Martin JL, Williams AJ, et al. Effect of latanoprost on the expression of matrix metalloproteinases and their tissue inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci 2006;47:9:3887-95.
7. Seal JR, Robinson MR, Burke J, et al. Intracameral sustained-release bimatoprost implant delivers bimatoprost to target tissues with reduced drug exposure to off-target tissues. J Ocular Pharm Ther 2019;35:1:50-57.
8. Weinreb RN, Lindsey JD, et al. Prostaglandin FP agonists alter metalloproteinase gene expression in sclera. Invest Ophthalmol Vis Sci 2004;45:4368-4377.
9. Yamada H, Yoneda M, Gosho M, et al. Bimatoprost, latanoprost, and tafluprost induce differential expression of matrix metalloproteinases and tissue inhibitor of metalloproteinases. BMC Ophthalmology 2016;16:26.
10. Perkumas K; Rhee DJ; Stamer DW. Differential effects of bimatoprost vs. bimatoprost free acid on outflow cell MMPs. Invest Ophthalmol Vis Sci 2021;62:551.
11. Richter M, Krauss AH, Woodward DF, Lütjen-Drecoll E. Morphological changes in the anterior eye segment after long-term treatment with different receptor selective prostaglandin agonists and a prostamide. Invest Ophthalmol Vis Sci. 2003;44:10:4419-26.
12. Yucel YH, Johnston meibomian gland, Ly T, et al. Identification of lymphatics in the ciliary body of the human eye: A novel “uveolymphatic” outflow pathway. Exp Eye Res 2009;89:5:810-819.
13. Tam AL, Gupta N, Zhang Z, et al. Latanoprost stimulates ocular lymphatic drainage: An in vivo nanotracer study. Trans Vis Sci Tech 2013;2:5:3.