As advances in reproductive technology enable pregnancies at increasingly older ages and new diagnostic tools allow for earlier disease detection, it’s becoming more common to see pregnant patients with glaucoma. Treating glaucoma in this patient population requires balancing the patient’s ocular health with any potential risks to the fetus from medications. Here, I’ll review the available evidence and discuss management options for pregnant patients.
IOP and Pregnancy
What do we typically expect in terms of intraocular pressure and pregnancy? Studies on IOP and pregnancy are limited, but the available data indicate that IOP changes occur throughout pregnancy as a result of an increase in the outflow facility.1 A study of 117 non-glaucomatous Nigerian women reported that intraocular pressure declined by the third trimester (mean IOP: 14.7 ±2.2 mmHg in the first trimester vs. 11 ±1.3 mmHg in the third trimester, p<0.0001).2 At six weeks postpartum, mean IOP increased to near pre-pregnancy levels at 14.2 ±1.8 mmHg (p<0.001). Another study reported that IOP decreased significantly by the 18th week of pregnancy, by almost 20 percent, in non-glaucomatous women, while ocular hypertensive women saw a later IOP decline of approximately 24 percent, at around the 24th week of pregnancy.3
 |
As for pregnant patients with glaucoma, a retrospective study of 15 pregnant women with ethnicities somewhat representative of the U.S. population,4 found that 57 percent of women had stable IOP and visual fields; 18 percent had visual field progression with IOP either stable or increased; and another 18 percent had stable visual fields with increased IOP. More than a third of the pregnant women in this small series had increased IOP or visual field progression during pregnancy. This tells us that we have to be vigilant about following and checking pressures and observing glaucomatous women during pregnancy.
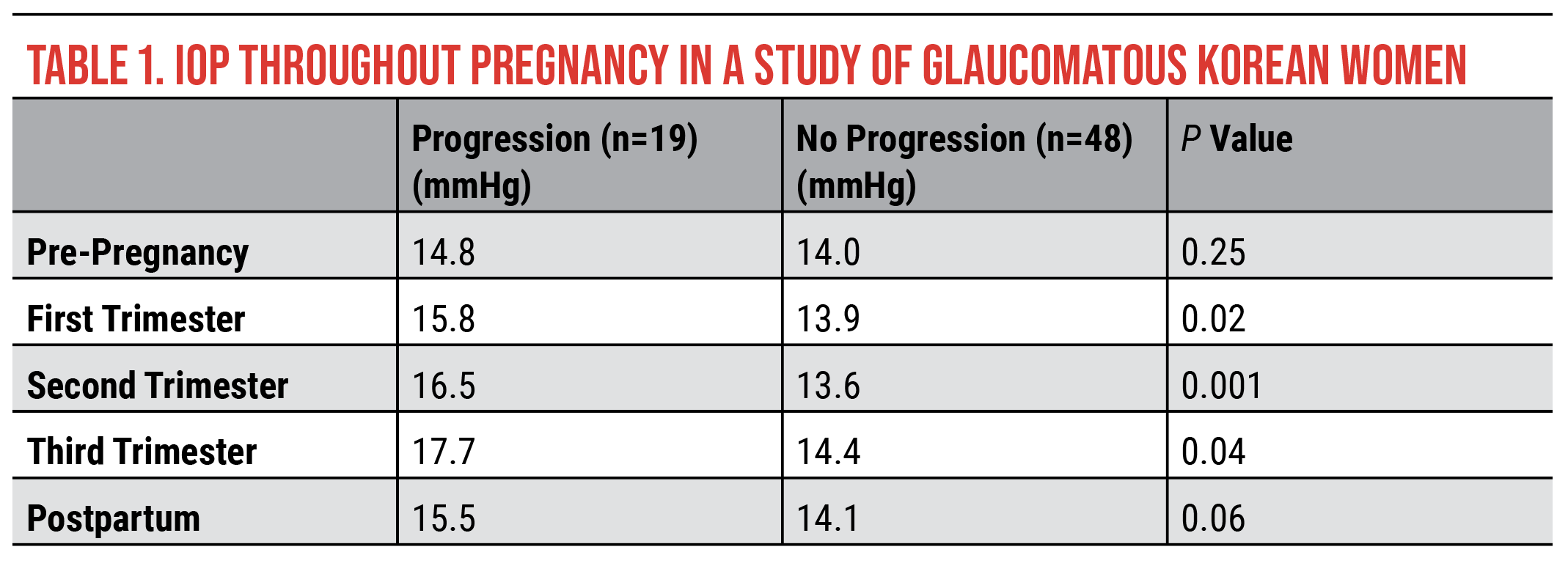
Considering these reported IOP changes during pregnancy, what happens after pregnancy in terms of disease progression? A retrospective study of 37 Korean women (67 eyes) who discontinued their glaucoma medications during pregnancy found that 28 percent—nearly a third of patients—had visual field progression detected 14 months after delivery.5 So, there seems to be a delay in the field progression despite IOP potentially increasing or increasing above where it had been during pregnancy.
This group compared patients who progressed with those who didn’t progress and found a statistically significant difference in IOP. Patients who progressed experienced higher IOP during pregnancy and postpartum compared with those who didn’t progress. Differences between the two groups were small, however, with progressors and non-progressors within 1 to 3 mmHg of each other (Table 1). Pressures in the study were also relatively low; this is because the study was conducted in Korea, and Korean women have a much higher incidence of normal-tension glaucoma than what we see in the United States (i.e., almost half of Korean women have normal-tension glaucoma). Even at these low pressures, almost a third of patients progressed following pregnancy.
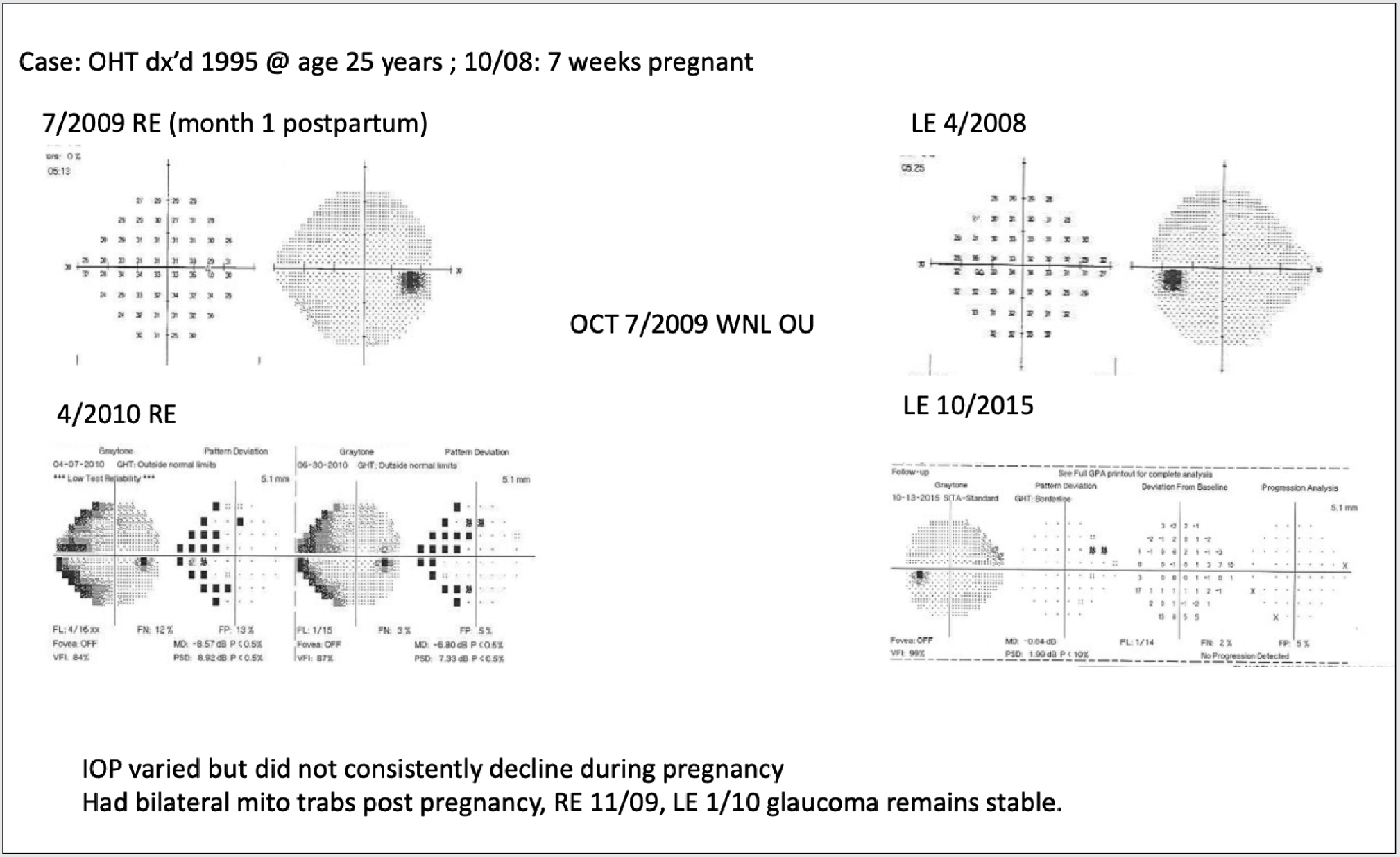
Case Example
This patient was diagnosed with ocular hypertension in 1995 when she was 25 years old. Thirteen years later in 2008, she came to me for a follow-up exam and was seven weeks pregnant. In 2009, her visual fields were full in the right eye, one month after she delivered. Left-eye visual fields were full in 2008. At the end of her pregnancy, her fields remained full and stable. Her OCTs were within normal ranges in both eyes. Within a year, by April 2010, the right eye had progressed to a dense nasal step, superior and inferior. Fortunately, the left eye remained full. During her pregnancy, her intraocular pressure varied but didn’t consistently decline. Based on her visual fields, I felt it was important to intervene. We performed bilateral mitomycin trabeculectomies, one in 2009 and in the second eye in 2010. |
Management Options
Pregnant patients can and do experience glaucomatous progression. Here are the key treatment options for managing their glaucoma:
• Laser trabeculoplasty. Laser trabeculoplasty, particularly selective laser trabeculoplasty has become a first-line treatment option for lowering IOP. That’s because the risk is fairly minimal. Topical anesthetics can be used and SLT can be performed without the use of any other pre- and postop anti-inflammatory medications (or if they’re needed, it’s for a very short period of time). So, laser trabeculoplasty isn’t a risky procedure for pregnant patients and should be considered as a first-line option. The biggest unknown is whether or not SLT is effective in young pregnant patients. Currently, there are few to no reports in the literature about SLT’s efficacy in this patient population. Further studies will provide more data.
• Medical management. Medical management is often the second treatment option for glaucoma patients. The FDA classifies available medications into risk categories for pregnancy, from risk category A (no risk) to category E (the most risk) and an unassigned group (Table 2). There are no class A glaucoma medications. Class B glaucoma medications include selective alpha-adrenergic agonists (brimonidine and apraclonidine)—animal studies suggest these drugs are safe, but there aren’t sufficient human studies to be totally confident.
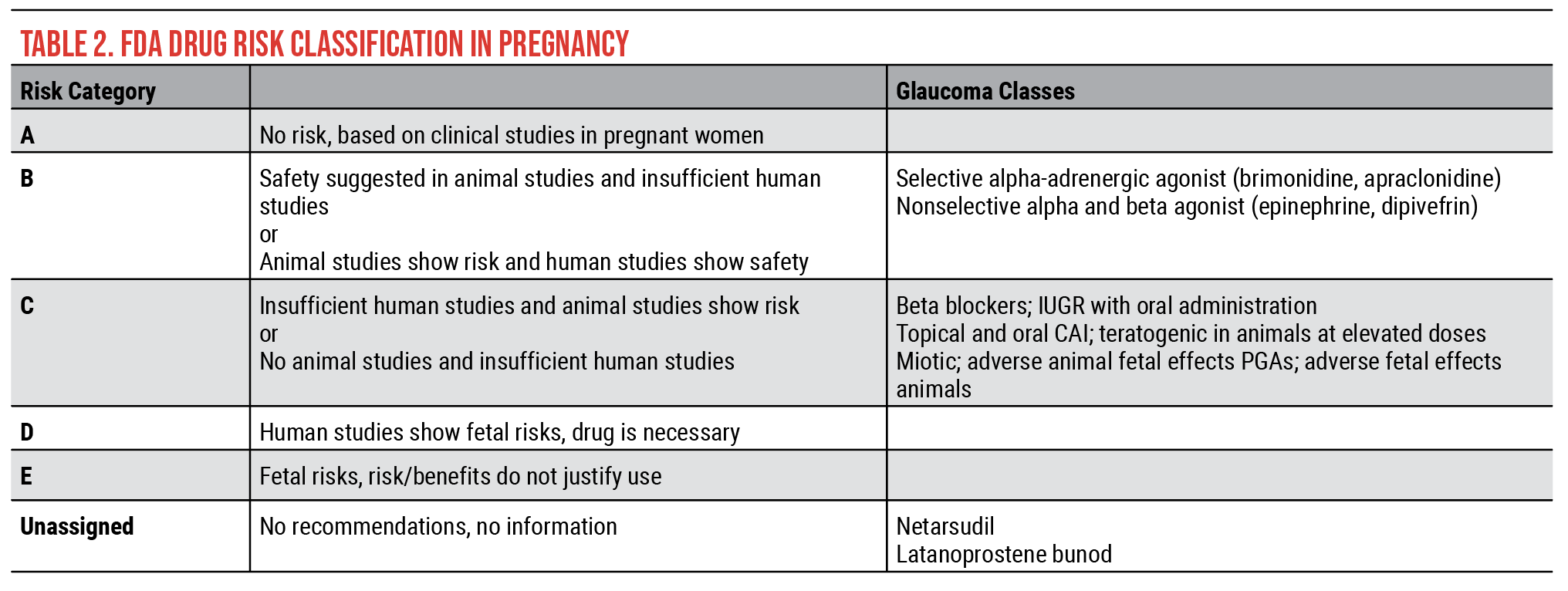 |
Most of the glaucoma medications are class C, which includes beta blockers, topical and oral carbonic anhydrase inhibitors, miotics and prostaglandin analogues. These are categorized as class C because there aren’t sufficient animal or human studies to definitively determine risk. Even though beta blockers are classified as risk category C drugs, we know that they’re used systemically to treat pregnancy-induced hypertension; if systemic use is safe then topical use should also be acceptable.
The unassigned category includes netarsudil and latanoprostene bunod. These are our two newest glaucoma medication classes. They’re unassigned because there isn’t enough information on their safety yet.
Generally, this is the regimen to be considered when using topical medications:
—The first trimester: brimonidine, beta blockers and prostaglandin analogues. Prostaglandin analogues are the most commonly prescribed class of compounds for glaucoma. The concern with prostaglandins is that they increase uterine tone and stimulate uterine contraction. However, the quantity that’s delivered after topical dosing is many magnitudes too low to cause uterine changes.
—The second trimester: brimonidine, beta blockers and prostaglandin analogues.
—The third trimester: beta blockers and prostaglandin analogues. A topical carbonic anhydrase inhibitor can be used or substituted for brimonidine. Carbonic anhydrase inhibitors shouldn’t be used earlier in the pregnancy if it can be helped since there have been some reports of limb abnormalities in small animals that received 20x the human dosage of oral carbonic anhydrase inhibitors. It’s unlikely to pose a risk in pregnant patients, but it may be preferable to avoid it.
—Nursing patients: beta blockers, topical carbonic anhydrase inhibitors and prostaglandin analogues. In nursing patients, we want to avoid brimonidine. This drug creates the greatest amount of risk for babies.
All medication should be used cautiously, particularly in the first trimester during organogenesis. It’s recommended to use the least amount of medication, especially in the first trimester, without adversely affecting the pregnant patient and her ocular status.
• Glaucoma surgery. This is the third treatment modality for pregnant glaucoma patients. There are some pregnancy-specific considerations, including anesthetics, body positioning, intraoperative drug use and choice of procedure.
Local anesthetics are a better option for pregnant patients than systemic anesthetics. Most glaucoma surgeries can be done topically with local anesthetics. It’s important to use them in small but sufficient quantities to anesthetize and keep the patient comfortable. Anesthetics considered safe during pregnancy are lidocaine and etidocaine, both of which fall in risk category B.
At the time of surgery, the glaucoma surgeon will work with the anesthesiologist to position the patient correctly on the table to avoid compressing the aorta or interior vena cava, which would be deleterious to the patient as well as the fetus. Positioning may include using pillows to support the patient’s back and knees and to ensure she isn’t folded over anteriorly.
Standard antimetabolites such as mitomycin-C and 5-FU cannot be used during surgery in pregnant patients as these are teratogenic and can adversely affect the fetus. Fortunately, there are many more surgical options now, such as MIGS. Typically short procedures, MIGS are a good option because they don’t require a patient to be in an uncomfortable position for a prolonged period, and the amount of anesthetics needed should be minimal.
For pregnant patients with advanced disease, a trabeculectomy is inadvisable due to concerns with mitomycin-C and 5-FU, but a tube implant under local anesthesia is an option. It will take slightly longer but can be performed if other surgical options such as MIGS have been exhausted.
C-section Delivery
There has been discussion on the American Glaucoma Society Net as to whether or not a C-section is indicated in pregnant women with glaucoma, particularly those who may have a thin filtering bleb. With a lack of studies on IOP changes during delivery in patients with glaucoma, we can instead look at IOP during labor and delivery in non-glaucomatous patients. This data, however, may or may not be indicative of what happens in glaucomatous patients during labor.
One study of 64 non-glaucomatous women (who had normal vaginal deliveries) found a small but statistically significant increase of less than 1.5 mmHg during labor but a decrease of 3 mmHg after labor.6 Another study of 30 non-glaucomatous women that also analyzed normal vaginal deliveries reported that patients who had epidural anesthesia showed no change in IOP (mean IOP: 11 to 12 mmHg) and no change in IOP immediately after contractions.7
Currently, there’s no clear answer to the C-section question because there’s no data that tell us whether or not there’s any risk to taking a pregnant glaucomatous patient through labor, particularly one with a thin bleb. Likewise, I haven’t come across any anecdotal reports of problems. The choice between having a C-section or spontaneous normal vaginal delivery is a difficult decision that needs to be made with the patient, the obstetrician and the glaucoma surgeon.
Postpartum Medications
After delivery, many mothers nurse their babies. There are some concerns with the medications that we use to treat glaucoma—for example, we know that beta blockers are actively secreted into breast milk.8 Timolol may have six times the plasma concentration after dosing topically, though it’s only 1/80th of a cardiac dose, so it’s small.
Betaxolol has a three times plasma concentration, but both non-selective and selective beta blockers can have potential adverse effects on a newborn baby such as apnea and bradycardia, as the drugs are transmitted into the breast milk and then to the baby.
We also know that the systemic drug level is highest within two hours after topical dosing. Thus, it’s recommended that if a patient is taking timolol eyedrops, she should dose after nursing as opposed to before nursing, or at least more than two hours before she plans to nurse.
Brimonidine may be secreted into breast milk. There have been reports in young children of apnea, hypertension and central nervous system depression, so this medication must be stopped during the third trimester when a patient is approaching delivery. Brimonidine should also most likely be avoided in nursing mothers. We know about the CNS depression, the apnea and hypotension in small children who have glaucoma who’ve been dosed with brimonidine, so there’s the concern that if it’s secreted into the breastmilk of a nursing mother, the side effects could occur in babies.
Cholinergic agonists aren’t commonly used in pregnant patients because of their side effect profiles, which may include headache, blurry vision, small pupil and change in refractive error. There have been anecdotal reports of hypothermia, seizures and restlessness in newborns.9 These are anecdotal reports, but even so, cholinergic agonists would probably not be one of the first drug classes prescribed and would more likely be used only if absolutely necessary.
Systemic and topical carbonic anhydrase inhibitors are both approved for use during lactation by the American Association of Pediatrics. We have data on their use during pregnancy and lactation because systemic carbonic anhydrase inhibitors are used to treat idiopathic intracranial hypertension in young women who are pregnant and following delivery. This history of systemic carbonic anhydrase inhibitor use seems to indicate that they’re reasonably safe to use during lactation.
We know that prostaglandin analogues are secreted in the breast milk of animals.10 In humans, a study of 11 pregnant women reported no adverse effects while treated with prostaglandin analogs during pregnancy and no effects in their newborns.10
An animal study that dosed rabbits with 80 times the amount of latanoprost that’d be given to human patients resulted in a quarter of the rabbits delivering non-viable fetuses.11 Importantly, the animals in these studies are far smaller than humans and have been given a much larger dose. Once again, the 11 pregnant women mentioned previously had no adverse effects from prostaglandin analogues.
We know that prostaglandin analogues can cross the blood-placenta barrier, but the plasma concentration following topical use is insufficient to affect prostaglandin analogue receptors in the uterus or elsewhere the body. A study using data from the US PharMetrics Plus database included 3,881 women aged 15 to 45 who were taking prostaglandin analogues and the 3,881 controls who were not taking prostaglandin analogues. The study authors found no significant difference between the number of spontaneous abortions that occurred in the two groups: 10 percent of the pregnant patients taking prostaglandin analogues had spontaneous abortions and 7 percent of those not taking prostaglandin analogues had spontaneous abortions (p=0.17).12
Nothing in the human literature suggests a reason to avoid using prostaglandin analogues during pregnancy. As best we can tell, they’re safe during pregnancy, during delivery and during nursing. This class of compounds has been our first line since the late 90s and remains a first-line treatment for pregnant patients as well as our other glaucoma patients.
 |
| Figure 1. Nasolacrimal occlusion is used to reduce systemic absorption of topical glaucoma medications through the lacrimal duct and blood vessels in the nose. The index finger of each hand is pressed against the medial corner of the eye for two minutes and the eyes are closed for two minutes. |
Reduce Systemic Absorption
How do we manage medications during and after pregnancy? With every patient, we want to reduce systemic absorption in order to have the fewest and least severe systemic side effects. This is especially important to emphasize to our pregnant patients with glaucoma.
To reduce systemic absorption, we ask patients to close their eyes for at least two minutes and occlude the nasolacrimal area for at least two minutes (Figure 1). Punctal plugs are an option for patients who can’t manage this and for those who might have systemic side effects from topical medications.
In summary, IOP findings in non-pregnant women may not always be generalizable to pregnant women. We don’t have the data to confirm or deny that statement. Intraocular pressure findings in non-glaucomatous pregnant women may also not be generalizable to glaucomatous pregnant women. Once again, we don’t have the data.
Don’t reduce the frequency of follow-up visits for pregnant patients, particularly those at high risk for glaucoma progression. We don’t know when these patients’ pressures are going to increase, how much they’re going to increase or how frequently they’re going to increase.
Be sure to carefully monitor and treat elevated IOP. Based on the information we have, if a patient’s IOP increases, it’s important to treat the increase to avoid seeing progression on the visual fields 12 to 14 months post-delivery.
When considering treatment options, use the laser, medical and surgical options that provide the lowest risk to the patient and to the fetus or the infant. We need additional studies to provide more robust data in pregnant and nursing patients so we can better design and inform our treatment protocols in this patient population.
Dr. Singh is a professor of ophthalmology and chief of the Glaucoma Division at Stanford University School of Medicine. He is a consultant to Alcon, Allergan, Santen, Sight Sciences, Glaukos and Ivantis.
Dr. Netland is Vernah Scott Moyston Professor and Chair at the University of Virginia in Charlottesville.
Dr. Serle is a Professor Emerita of Ophthalmology at the Icahn School of Medicine at Mount Sinai in New York. She reports financial relationships with AscelpiX, Kriya, Palatin and Qlaris.
1. Ziai N, Ory SJ, Khan AR, Brubaker RF. Beta-human chorionic gonadotropin, progesterone, and aqueous dynamics during pregnancy. Arch Ophthalmol 1994;112:801-806.
2. Ebeigbe JA, Ebeigbe PN, Ighoroje AD. Intraocular pressure in pregnant and non-pregnant Nigerian women. Afr J Reprod Health 2011;15:20-23.
3. Qureshi IA. Intraocular pressure and pregnancy. A comparison between normal and ocular hypertensive subjects. Arch Med Res 1997;28:397-400.
4. Brauner SC, Chen TC, Hutchinson BT, Chang MA, Pasquale LR, Grosskreutz CL. The course of glaucoma during pregnancy: A retrospective case series. Arch Ophthalmol 2006;124:1089-94.
5. Seo D, Lee T, Kim J, Seong G, Choi W, Bae H, Kim C. Glaucoma progression after delivery in patients with open-angle glaucoma who discontinued glaucoma medication during pregnancy. J Clin Med 2021;10:10:2190.
6. Avasthi P, Sethi P, Mithal S. Effect of pregnancy and labour on intraocular pressure. Int Surg 1976;61:82-84.
7. Meshi A, Armarnik S, Mimouni M, Segev F, Segal O, et al. The effect of labor on the intraocular pressure in healthy women. J Glaucoma 2017;26:59-64.
8. Lustgarten JS, Podos SM. Topical timolol and the nursing mother. Arch Ophthalmol 1983;101:9:1381–1382.
9. Samples JR, Meyer BS. Use of ophthalmic medications in pregnant and nursing women. Am J Ophthalmol 1988;106:5:616-23.
10. Vajaranant TS. Clinical dilemma—topical prostaglandin use in glaucoma during pregnancy. JAMA Ophthalmol 2022;140:6:637–638.
11. De Santis M, Lucchese A, Carducci B, Cavaliere AF, De Santis L, Merola A, et al. Latanoprost exposure in pregnancy. Am J Ophthalmol 2004;138:305–306.4.
12. Etminan M, Richter L, Sodhi M, et al. Association of topical prostaglandin analogue use with risk of spontaneous abortion. JAMA Ophthalmol 2022;140:6:634-636.