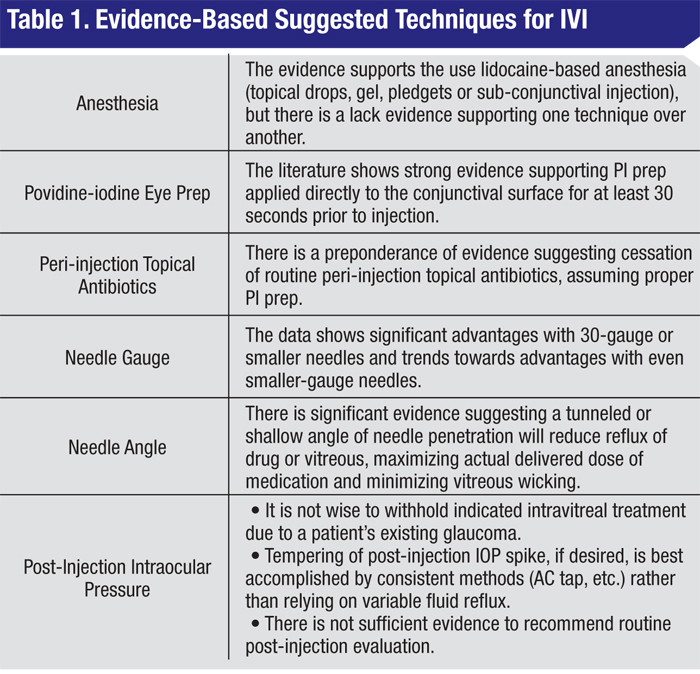
It has been a decade since Lloyd P. Aiello, MD, and colleagues published their consensus guidelines on the technical aspects of intravitreal injections.1 In 2004, only one agent was approved for intravitreal injection, and anti-VEGF agents had not yet been approved in ophthalmology. Since that time, intravitreal injections have become a cornerstone of retinal care and one of the most commonly performed procedures across all specialties. We have seen practices change dramatically and evidence on this topic mount. The 10-year anniversary of the guidelines is a good opportunity to examine the evidence in this area. Herein, we consider several of the critical technical aspects of IVI, broadly examining the literature and offering evidence-based suggestions based upon the available literature (See Table 1).
Anesthesia
Current techniques of pre-injection anesthetics vary widely and have been well-studied. In one randomized prospective trial of 24 patients, no difference in intravitreal injection pain scores was found between topical proparacaine and tetracaine, lidocaine pledget, and subconjunctival lidocaine for pre-injection anesthesia.2 Another randomized, prospective study of 28 patients showed significantly lower patient pain scores with subconjunctival 4% lidocaine versus topical 4% lidocaine for the IVI portion of the procedure; however, the combined pain score of the subconjunctival injection and IVI together was equivalent in both groups.3 Alternatively many retina surgeons employ lidocaine gel for pre-injection anesthesia. In a prospective, randomized study of 120 patients comparing proparacaine 0.5% drops, proparacaine + 4% lidocaine-soaked cotton tipped swabs held against the injection site for 20 seconds, and 3.5% lidocaine gel, no difference in pain scores was found between these methods.4 In another prospective, randomized study of 120 patients comparing topical lidocaine 2% gel and subconjunctival lidocaine 2% injection, they found no difference in pain scores.5 Based on this evidence, we suggest one of the above lidocaine-based methods, but feel there is a lack of evidence to support recommending one technique over another.
Povidine-iodine Eye Prep
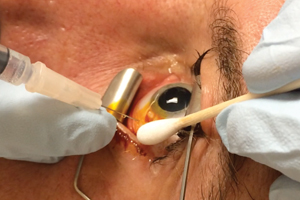
Providone-iodine is the mainstay of conjunctival antibacterial surface preparation and is widely considered part of the standard of care for IVI (See Figure 1). Typically, 5% PI solution is used. There are two additional important issues in this area: 1) the length of time the PI should be in contact with the conjunctiva; and 2) the order in which anesthetic gel and PI should be applied.
|
One laboratory study looking at common pathogens in endophthalmitis showed that bacterial kill time for PI ranges from 30 to 120 seconds, and that 15 seconds was inadequate.6 In another study, exposure to 5% PI for 15 seconds failed to yield significant reduction in conjunctival bacteria, with only a 42-percent reduction in bacteria; significant reduction was observed after 30 seconds.7 In another study, 5% PI showed 96.7-percent bacterial kill rates achieved with 60 seconds of contact. The authors also note that 5% PI is more effective than 1% PI.8
One laboratory study showed that if the PI was placed on culture plates before the lidocaine gel, the bacterial counts were equivalent to PI only (no growth); however, if PI was placed onto the plates after the lidocaine gel, bacterial growth was similar to plates which had no PI applied at all.9 Another laboratory study demonstrated that applying lidocaine gel before 5% PI severely decreased effectiveness against common endophthalmitis pathogens versus 5% PI in direct contact.10 We feel the literature shows strong evidence supporting application of PI directly to the conjunctival surface for at least 30 seconds after administration of aqueous topical anesthetic but prior to administration of gel, if used.
Interestingly, a recent study incubated common endophthalmitis pathogens with and without lidocaine 2%/methylparaben 0.1% and showed a 90- to 95-percent reduction in colonies in the lidocaine group, suggesting an antibacterial effect of this agent. The authors also presented a chart review of IVIs either with subconjunctival lidocaine or without; they found a statistically significant reduction in endophthalmitis rates in those with subconjunctival lidocaine.11
Peri-injection Topical Antibiotics
In 2004, Dr. Aiello and colleagues refrained from recommending peri-IVI antibiotics, due to lack of evidence.1 Ten years later, there has been sufficient study of routine peri-injection antibiotic use to suggest that they should not be considered standard of care and may preferably be avoided.
In 2004, Dr. Aiello and colleagues refrained from recommending peri-IVI antibiotics, due to lack of evidence.1 Ten years later, there has been sufficient study of routine peri-injection antibiotic use to suggest that they should not be considered standard of care and may preferably be avoided.
|
No incremental benefit of peri-IVI antibiotics has been shown, if PI is appropriately used for prep.16 Also, neither topical moxifloxacin nor gatifloxacin achieves therapeutic levels in the vitreous. (Costello P. Vitreous penetration of moxifloxacin and gatifloxacin after topical administration in humans. Paper presented at Annual meeting of the American Academy of Ophthalmology October 23, 2004; New Orleans.) Additional cost for this common procedure is also an issue. We feel there is a preponderance of evidence suggesting the avoidance of routine peri-IVI topical antibiotics, assuming proper PI prep is employed.
Needle Diameter
With respect to needle size, three important potential complications have been investigated: vitreous incarceration; fluid reflux; and patient discomfort for needles ranging from 27- to 32-ga.
• Vitreous incarceration. Vitreous incarceration (wick) may be a risk factor for endophthalmitis.17 In a laboratory ex vivo study, 32-ga. needles demonstrated less vitreous incarceration at the tract site than either 27- or 30-ga. needles (p<0.01) with endoscopic investigation.18
• Fluid reflux. Fluid backflow through the needle tract may result in reduced volume of drug delivery.19 Laboratory studies have shown that 27-ga. needles are associated with the formation of subconjunctival blebs or reflux post IVI at a greater rate than 30- or 32-ga. needles.18,20
• Patient discomfort. In one survey study of 60 IVI patients comparing 27-, 30 and 32-ga. needles, the gauge of the needle was not found to affect pain scores to a statistically significant level.21 However, in another study, statistically significant lower pain scores were found with smaller gauges, comparing 26- or 27-ga. needles to 29- and 30-ga. needles (p<0.001).22
Importantly, no reports of significant disadvantages of smaller gauge needles have been published. However, reduced fluid reflux associated with smaller needles likely leads to higher post-injection intraocular pressure elevations compared with larger needles; this important point is discussed below. We feel the data shows significant advantages with 30-ga. or smaller needles and trends towards advantages with even smaller-gauge needles for IVI.
Needle Angle
The angle of penetration of the needle in relation to the sclera is an important, but often overlooked, aspect of IVI. Significant variation of fluid reflux has been shown with various needle angles. The consequence of fluid reflux may be reduced effective medication dose, vitreous wicking and IOP fluctuation. For proper perspective, a well-structured rabbit study evaluated reflux using PET/CT after IVI of I-124-labelled anti-VEGF. Straight perpendicular injection with a 32-ga. needle with a cotton swab placed as the needle was withdrawn was used in this protocol. Immediately following injection, each subject was imaged with micro PET/CT. The subconjunctival bleb at the injection site was clearly visible by PET/CT and therefore contained the labeled anti-VEGF, showing that drug does reflux—in this experimental setting.19
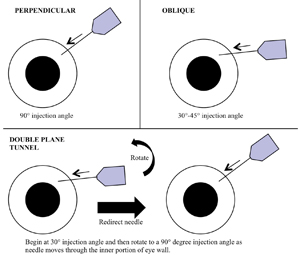
In a study of 105 patients randomly assigned to either straight perpendicular (90°), shallow oblique (30° to 45°), or double-plane tunnel injection (beginning at 30° and redirecting to 90°), the occurrence of fluid reflux was recorded (See Figure 2). In the straight injection group, 51.4 percent of patients had reflux; 34.3 percent did so in the oblique group, and only 17.1 percent did so in the double-plane tunnel group.23 In another non-randomized prospective study of 88 eyes, the mean measured reflux bleb was statistically less with a double-plane tunneled injection versus eyes undergoing the straight perpendicular injection (p<0.001).24 Another randomized study of 60 patients comparing straight or tunneled needle paths noted no difference in IOP after five minutes and equivalent patient pain scores between groups; however, less reflux was found in the tunneled group.25
We feel there is significant evidence suggesting that a tunneled or shallow needle angle will reduce reflux of drug or vitreous back through the sclerotomy, maximizing actual delivered dose of medication and minimizing wicking. Of note, the amount and occurrence of fluid reflux with any needle angle is inconsistent and variable between patients (17 to 51 percent, shown above). As further discussed below, if IOP modulation is deemed beneficial to a patient, we feel that more consistent IOP-modulating methods should be employed and the needle angle which appears to reduce uncontrolled fluid reflux (either drug or vitreous) should be chosen.
Post-Injection IOP
Significant, although short-lived, elevation in intraocular pressure occurs after IVI, even with 0.05 ml of injection volume. The long-term effects of this transient elevation are not known, though there may be risk of late prolonged intraocular elevation.26,27 In patients with advanced glaucoma or other optic neuropathy, special consideration may be warranted.
 |
Needle path affects post-IVI intraocular pressure. In 45 eyes, the mean IOP in eyes receiving a standard, straight scleral incision was 21.9 ±14.2 mmHg (median 22.3) versus 33.5 ±7.2 mmHg (median 34.7) in the tunneled scleral incision group (p=0.001).30 However, as the occurrence of reflux is variable between patients and the reflux may consist of drug, we do not recommend using a straight needle path in an attempt to mitigate IOP spike.
Another study examined post-IVI IOP with or without application of moderate pressure to the globe with cotton swab before injection (for anesthetic purposes). The authors found this technique lowered the mean IOP change immediately after injection, with 35 percent of eyes without pre-injection pressure having post-injection IOP ≥50 mmHg compared with only 10 percent of eyes that had pre-injection pressure (p<0.001).31
At this time, long-term effects of frequent/monthly IOP spikes are not known. However, in patients in whom vision may be more sensitive to IOP insult, employing consistent techniques to mitigate IOP spikes may be advisable, such as pre-injection anterior chamber paracentesis. Relying on fluid reflux to mitigate IOP spikes is not advisable due to the variability of the reflux volume and the possibility that refluxed fluid is drug. Therefore, we suggest using a small-gauge needle and an oblique needle angle, thus minimizing reflux and assuring delivered dose and employing other methods of IOP mitigation, if desired.
An editorial by Dennis P. Han, MD, and Dale K. Heuer, MD, stated, “If intravitreal therapy is deemed appropriate, the physician should proceed with the knowledge that its risks are manageable and that visual outcome, not IOP, should be the final arbiter in the decision-making process.”32 We agree it is not wise to withhold indicated intravitreal treatment due to a patient’s existing glaucoma.
Interestingly, a recent survey of retinal specialists showed that nearly 30 percent do not perform immediate post-IVI ocular assessment,33 and we expect this number to decrease as injections become safer and our knowledge base increases. We feel there is not sufficient evidence to recommend routine post-injection evaluation.
With the advent of ocular anti-VEGF agents we have seen dramatic improvements in treatment of retinal disease. With continued study of IVIs, the goal must be to continually improve methods of drug delivery and provide even safer and more efficacious treatments with these medications. We encourage ophthalmologists to carefully consider the work done in this area and rely on evidence to guide practice, rather than habit.
REVIEW
Dr. Karth is a vitreoretinal fellow at the Byers Eye Institute, Stanford University. Dr. Blumenkranz is the chairman of the Department of Ophthalmology and a professor of ophthalmology at the Byers Eye Institute. Contact Dr. Karth at pkarth@stan ford.edu; (815)-355-5409; Byers Eye Institute, 2452 Watson Ct., Palo Alto, Calif. 94303.
1. Aiello LP, Brucker AJ, Chang S, Cunningham ET Jr, et al. Evolving guidelines for intravitreous injections. Retina 2004;24(5 Suppl):S3-19.
2. Blaha GR, Tilton EP, Barouch FC, Marx JL. Randomized trial of anesthetic methods for intravitreal injections. Retina 2011;31:535-539.
3. Kaderli B, Avci R. Comparison of topical and subconjunctival anesthesia in intravitreal injection administrations. Eur J Ophthalmol 2006;16:718-721.
4. Davis MJ, Pollack JS, Shott S. Comparison of topical anesthetics for intravitreal injections: A randomized clinical trial. Retina 2012;32:701-705.
5. Friedman SM, Margo CE. Topical gel vs subconjunctival lidocaine for intravitreous injection: A randomized clinical trial. Am J Ophthalmol 2006;142:887-888.
6. Berkelman RL, Holland BW, Anderson RL. Increased bactericidal activity of dilute preparations of povidone-iodine solutions. J Clin Microbiol 1982;15:635-639.
7. Friedman DA, Mason JO, Emond T, McGwin G. Povidone-iodine contact time and lid speculum use during intravitreal injection. Retina 2013;33:975-981.
8. Ferguson AW, Scott JA, McGavigan J, Elton RA, McLean J, Schmidt U, et al. Comparison of 5% povidone-iodine solution against 1% povidone-iodine solution in preoperative cataract surgery antisepsis: A prospective randomised double blind study. Br J Ophthalmol 2003;87:163-167.
9. Doshi RR, Leng T, Fung AE. Povidone-iodine before lidocaine gel anesthesia achieves surface antisepsis. Ophthalmic Surg Lasers Imaging 2011;42:346-349.
10. Boden JH, Myers ML, Lee T, Bushley DM, Torres MF. Effect of lidocaine gel on povidone-iodine antisepsis and microbial survival. J Cataract Refract Surg 2008;34:1773-1775.
11. Tustin A, Kim SJ, Chomsky A, Hubbard GB, Sheng J. Antibacterial properties of 2% lidocaine and reduced rate of endophthalmitis after intravitreal injection. Retina 2013; epub Oct 7.
12. Kim S, Toma H. Antimicrobial resistance and ophthalmic antibiotics: 1-year results of a longitudinal controlled study of patients undergoing intravitreal injections. Arch Ophthalmol 2011;129:1180-1188.
13. Milder E, Vander J, Shah C, Garg S. Changes in antibiotic resistance patterns of conjunctival flora due to repeated use of topical antibiotics after intravitreal injection. Ophthalmology 2012;119:1420-1424.
14. Dave SB, Toma HS, Kim SJ. Ophthalmic antibiotic use and multidrug-resistant staphylococcus epidermidis: A controlled, longitudinal study. Ophthalmology 2011;118:2035-2040.
15. Dave SB, Toma HS, Kim SJ. Changes in ocular flora in eyes exposed to ophthalmic antibiotics. Ophthalmology 2013;120:937-941.
16. Moss JM, Sanislo SR, Ta CN. A prospective randomized evaluation of topical gatifloxacin on conjunctival flora in patients undergoing intravitreal injections. Ophthalmology 2009;116:1498-1501.
17. Chen SD, Mohammed Q, Bowling B, Patel CK. Vitreous wick syndrome—a potential cause of endophthalmitis after intravitreal injection of triamcinolone through the pars plana. Am J Ophthalmol 2004;137:1159-60.
18. Hubschman JP, Coffee RE, Bourges JL, Yu F, Schwartz SD. Experimental model of intravitreal injection techniques. Retina 2010;30:167-173.
19. Christoforidis J, Williams M, Epitropoulos F, Knopp M. Subconjunctival bleb that forms at the injection site after intravitreal injection is drug, not vitreous. Clin Experiment Ophthalmol 2013;41:614-615.
20. Cortez RT, Ramirez G, Collet L, Thakuria P, Giuliari GP. Intravitreous bevacizumab injection: an experimental study in New Zealand white rabbits. Arch Ophthalmol 2010;128:884-887.
21. Rifkin L, Schaal S. Factors affecting patients’ pain intensity during in office intravitreal injection procedure. Retina 2012;32:696-700.
22. Rodrigues E, Grumann A, Penha F, Shiroma H, et al. Effect of needle type and injection technique on pain level and vitreal reflux in intravitreal injection. J Ocul Pharmacol Ther 2011;27:197-203.
23. Özkaya A, Alkin Z, Celik U, Yüksel K, Ozgurhan EB, Agca A, et al. Comparing the effects of three different intravitreal injection techniques on vitreous reflux and intraocular pressure. J Ocul Pharmacol Ther 2013;29:325-329.
24. Rodrigues EB, Meyer CH, Grumann A, Shiroma H, Aguni JS, Farah ME, et al. Tunneled scleral incision to prevent vitreal reflux after intravitreal injection. Am J Ophthalmol 2007;143:1035-1037.
25. Knecht PB, Michels S, Sturm V, Bosch MM, Menke MN. Tunnelled versus straight intravitreal injection: Intraocular pressure changes, vitreous reflux, and patient discomfort. Retina 2009;29:1175-1181.
26. Hoang QV, Jung JJ, Mrejen S, Freund KB. Influence of axial length and postinjection reflux on sustained intraocular pressure elevation as a result of intravitreal anti-vascular endothelial growth factor therapy. Retina 2013; Nov 14 epub.
27, Mathalone N, Arodi-Golan A, Sar S, Wolfson Y, Shalem M, Lavi I, Geyer O. Sustained elevation of intraocular pressure after intravitreal injection of bevacizumab in eyes with neovascular age-related macular degeneration. Graefe’s Arch Clin Exp Ophthalmol. 2012;250:1435-1440.
28. Arikan G, Osman Saatci A, Hakan Oner F. Immediate intraocular pressure rise after intravitreal injection of ranibizumab and two doses of triamcinolone acetonide. Int J Ophthalmol 2011;4:402-405.
29. Sharei V, Höhn F, Köhler T, Hattenbach LO, Mirshahi A. Course of intraocular pressure after intravitreal injection of 0.05 mL ranibizumab (Lucentis). Eur J Ophthalmol 2010;20:174-179.
30. Höhn F, Mirshahi A. Impact of injection techniques on intraocular pressure (IOP) increase after intravitreal ranibizumab application. Graefe’s Arch Clin Exp Ophthalmol 2010;248:1371-1375.
31. Gregori NZ, Weiss MJ, Goldhardt R, Schiffman JC, Vega E, Mattis CA, et al. Ocular Decompression With Cotton Swabs Lowers Intraocular Pressure Elevation After Intravitreal Injection. J Glaucoma. 2013 Apr 29. [Epub ahead of print]
32. Han DP, Heuer DK. Intravitreal corticosteroid therapy: Putting the problem of glaucoma in perspective. Arch Ophthalmol 2012;130:380-382.
33. Green-Simms AE, Ekdawi NS, Bakri SJ. Survey of intravitreal injection techniques among retinal specialists in the United States. Am J Ophthalmol 2011;151:329-332.