Restoring vision through cataract surgery remains a gift ophthalmologists provide patients, and the outcomes continue to improve. While a rare occurrence, post-cataract endophthalmitis occurs in a few thousand people each year in the United States and is a significant source of visual morbidity.
Here, we highlight the risk factors, prophylaxis, clinical presentation and treatment of post-cataract endophthalmitis.
Epidemiology
A retrospective population-based cohort study based on the Medicare claims database from 2006 to 2011 reported post-cataract surgery endophthalmitis incidence rates ranging from 0.06 to 0.20 percent.1 A more recent retrospective cohort study based on the American Academy of Ophthalmology Intelligent Research In Sight Registry database reported an incidence of 0.04 percent among patients 44 years and older.2 This study also found that PCE seems to occur at higher rates in younger patients, with an incidence of 0.37 percent in patients under 17 and 0.18 percent in those aged 18 to 44.
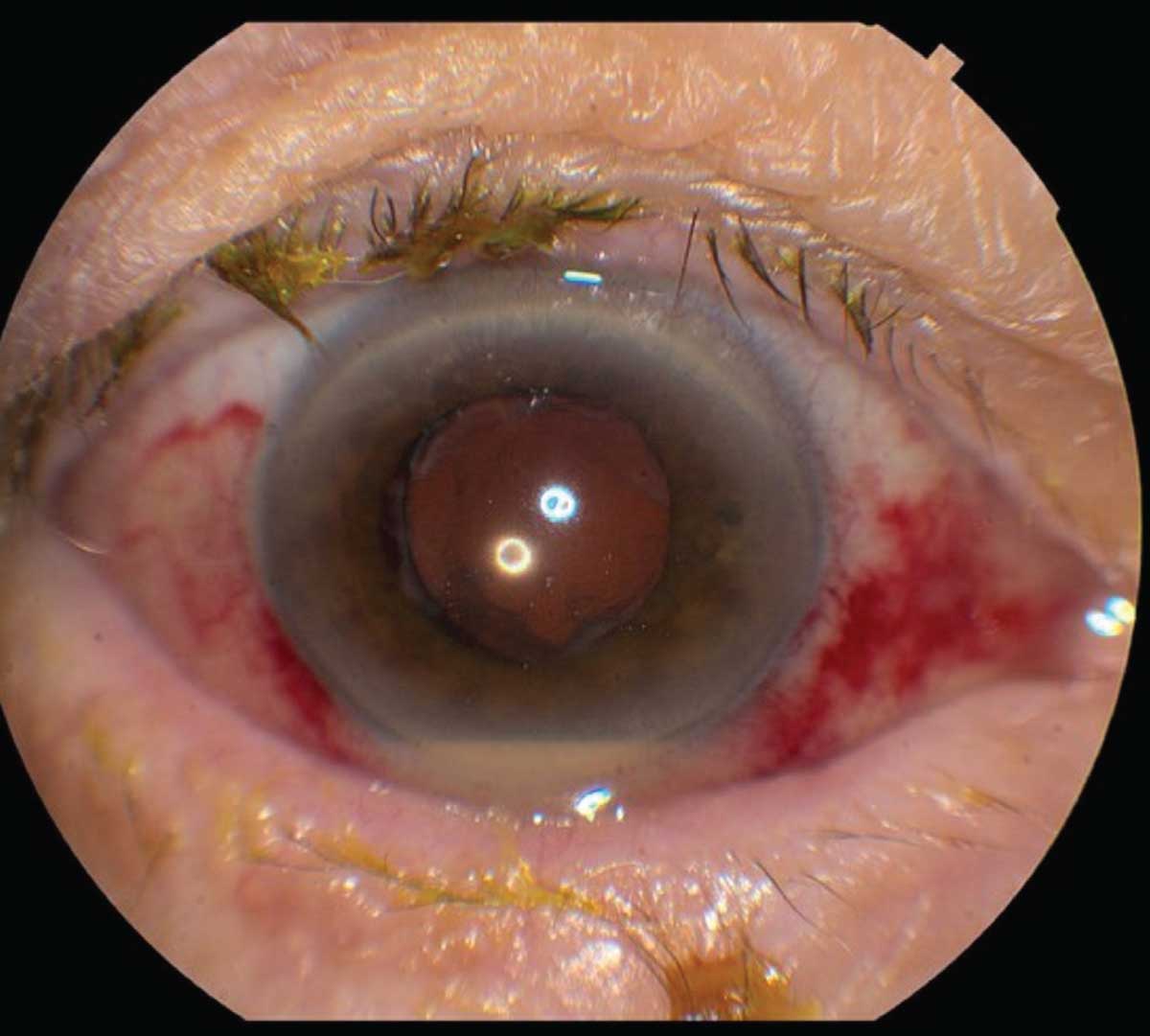 |
| Figure 1. This eye developed pain and decreased vision four days after uneventful cataract surgery. The subconjunctival hemorrhage is from the tap-and-inject procedure. Conjunctival injection and hypopyon are visible. |
Clinical Presentation
By definition, acute post-cataract endophthalmitis occurs within six weeks of surgery, typically within a few days, while cases that occur more than six weeks after surgery are deemed chronic endophthalmitis.3,4 Most patients present with symptoms including blurred vision (93.1 percent of cases), redness (80.6 percent), pain (75.4 percent), and eyelid swelling (33.1 percent).4 On examination, eyes often have a conjunctival injection. The anterior chamber shows inflammatory cell, hypopyon, flare and fibrin, and posterior synechia may be present (Figure 1). In the posterior segment, vitritis often obscures the fundus, and sometimes retinal hemorrhages occur. Significant media opacity often requires ultrasonography to grossly examine the posterior segment (Figure 2). In chronic cases, patients may present with indolent, persistent uveitis that can be mistaken for recurrent postoperative inflammation.5 Patients with chronic endophthalmitis often have milder symptoms than acute cases, and eyes may have initially responded to topical steroids before getting worse. Some findings suggestive of a chronic PCE include granulomatous precipitates on the cornea and intraocular lens without an obvious hypopyon, as well as the presence of white intracapsular plaques.5
Risk Factors
Risk factors can be divided into preoperative and intraoperative risk factors. Preoperative risks include age below 44 years old, male sex, living in a rural area and immunosuppressive conditions such as diabetes mellitus.2,3 Acute or chronic eyelid inflammatory disease can also be a risk factor.6 Intraoperative risk factors include vitreous loss, posterior capsular rupture, the need for an anterior vitrectomy, poor corneal wound construction and potentially the use of silicone or polymethyl methacrylate intraocular lenses rather than acrylic.3,6
Microbiology
Acute PCE can be caused by a diverse group of organisms. Gram-positive, coagulase-negative staphylococci are the most common, making up about 70 percent of culture-positive cases.7,8 Other common organisms include other Gram-positive cocci such as Staphylococcus aureus (6.8 to 10.2 percent), as well as Streptococcus (8.2 to 11.5 percent) and Enterococcus species (2.5 to 6.8 percent).7,8 Cutibacterium acnes, the organism formerly known as Propionibacterium acnes, is a slow-growing, facultative anaerobic, Gram-positive bacteria that can cause chronic postoperative endophthalmitis.9 Gram-negative bacteria make up about 5 percent of cases, with some notable species being Klebsiella pneumonia, Pseudomonas aeruginosa and Enterobacter species.3,7,8 Klebsiella pneumonia is especially prevalent in older populations and in Southeast Asia.3 Rarely, endophthalmitis can be caused by fungal organisms such as Candida albicans and Aspergillus species. The Endophthalmitis Vitrectomy Study found that approximately one-third of cases were culture negative. (This was the case even with newer diagnostic techniques.10)
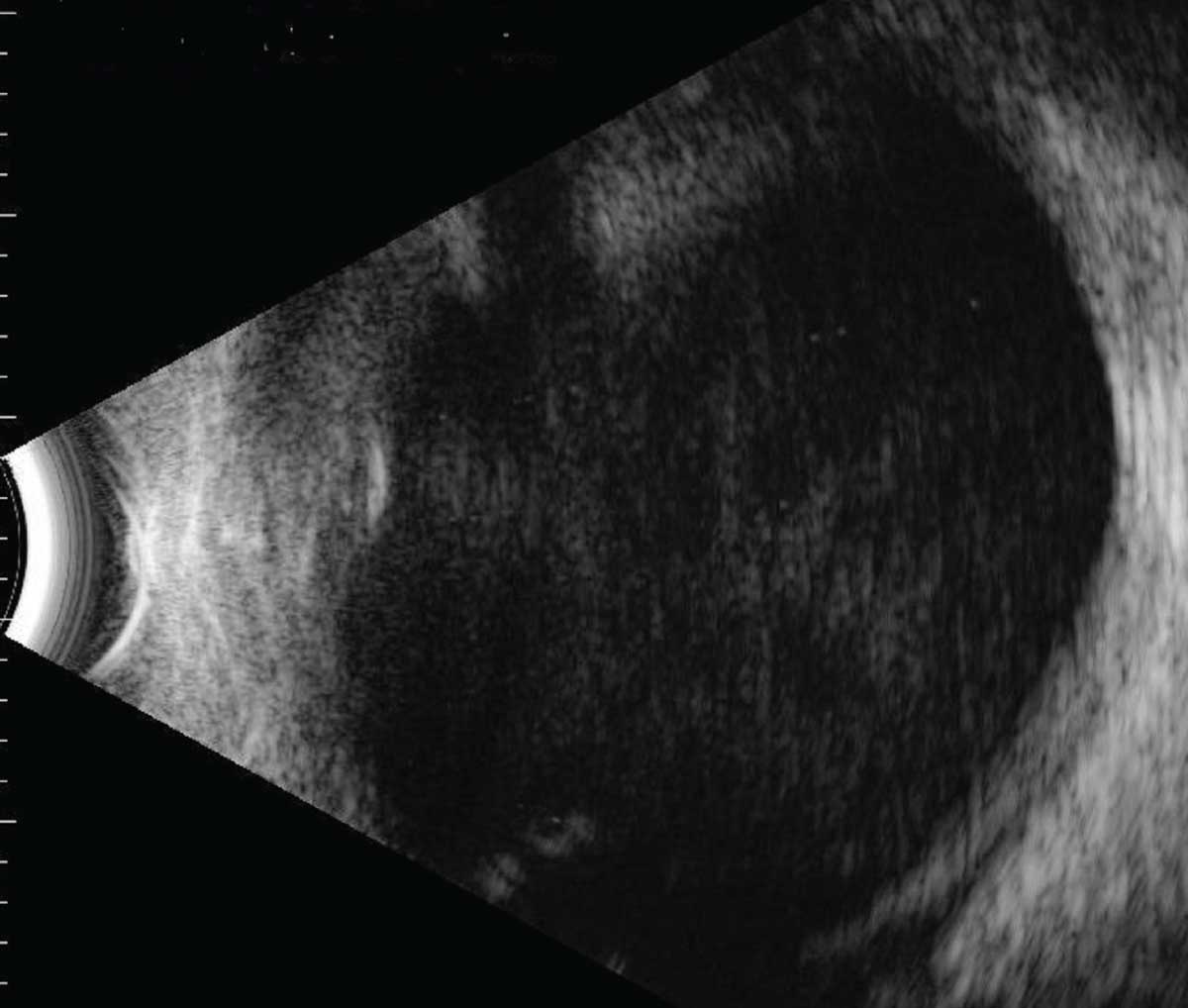 |
| Figure 2. B-scan ultrasonography demonstrating significant vitreous debris in an eye with acute endophthalmitis. |
Diagnostics
Ideally, identifying the causative organism could help tailor the antimicrobial used, and might give some idea about prognosis (for example, streptococcus and Gram-negative organisms generally have poor outcomes). Although intraocular samples are desirable, in some cases there may not be a reliable laboratory to process the specimen. In a recent retrospective review of 111 cases of post-cataract endophthalmitis, nine cases had a change in management based on clinical findings, but none changed based on microbiology. Cultures are helpful for guiding prognosis but may not be critical to management.11
Nonetheless, the current standard practice is to culture the vitreous from a vitreous tap procedure. However, sometimes you can’t obtain a vitreous tap. An anterior chamber tap is often easy to obtain but has a lower yield rate. The diagnostic yield of an AC tap is only 32.11 percent, compared to 64.74 percent for a vitreous tap.12 Clearly, there’s room for improvement in the sensitivity of diagnostic testing.
Polymerase chain reaction testing of samples may improve diagnostic yield. A prospective, randomized, multicenter cataract surgery study showed that PCR was able to increase detection rates from 48.3 percent, using standard cultures from vitreous tap, to 65.55 percent with PCR.13 PCR screening has been shown to have high sensitivity, specificity and positive predictive values.13 In certain laboratories, PCR also can yield results quickly—in a matter of hours, in contrast to several days by traditional culture techniques. An added benefit to PCR in the context of PCE is the potential to run a PCR screen on a sample obtained after administration of post-intravitreal antibiotics—a situation known to be high risk for false-negative culture results.
While PCR has these distinct advantages over culture, there are two main drawbacks to PCR. First is a lack of access, as many ophthalmology clinics can’t easily get samples to labs with rapid PCR technology. Second, one of the inherent limitations of PCR is that results are possible only if the causative organism’s genome is on the list of organisms screened by the test.
A new methodology called whole-genome-sequencing is being investigated for pathogen detection. With WGS, it’s possible to screen for all non-human DNA in the sample, making it a powerful tool for identifying causative organisms in PCE. WGS also provides a distinct advantage in that, unlike PCR, it doesn’t require a pre-existing list of potential pathogen genomes to detect the presence of foreign DNA. In theory, this would greatly increase sensitivity for detecting known PCE-causing pathogens, while also opening the door for the discovery of new causative organisms.
One study compared traditional culture to PCR and a WGS method called Biome Representational in silico Karyotyping (BriSK).10 The study found that traditional culture remained the gold standard, and a number of presumed endophthalmitis cases didn’t have detectable bacterial DNA. Interestingly, this study also found Torque Teno Virus (TTV) was present in 8/14 (57.1 percent) of the culture-positive samples and 7/7 (100 percent) of the culture-negative samples. This is a single-stranded DNA anellovirus that causes a panuveitis called SHAPU (Seasonal Hyper Acute Panuveitis), a condition that looks like a lot like acute endophthalmitis.10 A follow-up prospective cohort study investigated clinical outcomes in relation to the detected pathogen DNA detected by WGS, culture and qPCR.14 In this study, there was more variability in identifying causative organisms between the diagnostic methods. TTV was present in 23/47 (49 percent) samples. Patients with higher TTV viral load were 5.2 times more likely to need subsequent pars plana vitrectomy due to complications like retinal detachment and visually significant vitreous opacities.14 These studies highlight the clinical promise that WGS provides in pathogen detection. While this method is currently limited by access, labor and costs, WGS may help detect pathogens that can impact patient prognosis.
PCE-Prophylaxis
Though much work has been done on endophthalmitis prophylaxis, povidone-iodine remains the primary way to prevent post-procedure endophthalmitis. A non-randomized, prospective study showed that preoperative povidone-iodine reduced rates of culture-positive endophthalmitis compared to using silver protein solution (0.06% to 0.24%, p<0.03).15 Subsequent prospective studies showed that preoperative povidone-iodine is effective at reducing conjunctival flora, indicating that the agent can be an effective preoperative prophylaxis.16,17 However, even after administration of topical povidone-iodine, up to 25 percent of cases still have positive cultures.
The use of intraoperative intracameral antibiotic injection is increasingly used as prophylaxis against PCE. In a well-known analysis, the European Society of Cataract and Refractive Surgery (ESCRS) endophthalmitis study group conducted a multicenter, prospective, randomized clinical trial of 16,603 patients that investigated the clinical outcomes of intracameral cefuroxime and perioperative topical levofloxacin.18 They found that cohorts without intracameral cefuroxime were associated with a 4.92-times increased risk of developing PCE. On the other hand, there was no statistical difference in the rates of endophthalmitis with or without the application of levofloxacin.
Other large retrospective studies, using varying intracameral antibiotics including cefuroxime, moxifloxacin and vancomycin, found a reduction in endophthalmitis in cohorts that received intracameral injections.19,20,21 Additionally, one study supported the idea that post-surgery topical gatifloxacin or ofloxacin didn’t reduce endophthalmitis rates more than intracameral antibiotics alone.21
While the data favor intraoperative intracameral antibiotic prophylaxis, some uncertainty remains regarding what antibiotic agent is best. Cefuroxime was effective in the original ESCRS study, but reports of increasing rates of cefuroxime-resistant pathogens, especially Enterococcus species, have raised concerns that this agent may not be best for widespread prophylactic use.22 Other intracameral antibiotics, namely moxifloxacin and vancomycin, have been used as potential prophylactic agents. While intracameral moxifloxacin has been reported to be safe for prophylaxis,23 it’s still important to consider the possible complications and ocular side effects associated with these fluoroquinolone antibiotics. Systemic fluoroquinolones are associated with a pigment dispersion-like condition called bilateral acute iris transillumination (BAIT) syndrome, and a recent case report showed that unilateral BAIT-like syndrome can develop following intracameral moxifloxacin in the affected eye.24 The frequency of this adverse effect is yet to be determined, but it could increase as the use of intracameral moxifloxacin becomes more widespread.
The use of intracameral vancomycin also carries the risk of the rare but potentially devastating hemorrhagic occlusive retinal vasculitis, as reported in some retrospective case series.25, 26 HORV can appear one to 21 days after surgery. The visual outcomes are poor, with final vision reported as 20/100 or worse.25, 26 Due to the rare occurrence of HORV, there’s no absolute contraindication to the use of intracameral vancomycin, but its risks and benefits must be carefully considered.
Even with the rise of intracameral antibiotic use, topical antibiotics are still commonly used in the perioperative period. However, the efficacy of topical antibiotics remains controversial. The ESCRS study and another study have shown that there doesn’t seem to be a significant difference in infection rates when postoperative topical antibiotics such as gatifloxacin, ofloxacin and levofloxacin are used along with intraoperative intracameral antibiotics.18,21
As for preoperative topical antibiotics, a prospective, randomized, controlled, single-masked study with 46 patients showed that preoperative antibiotics prophylaxis was effective at reducing the flora of the conjunctiva, based on samples taken from swabbing the conjunctiva an hour before the surgery.27 However, it’s unclear whether this outcome translates to reduced rates of postoperative endophthalmitis, as a later prospective epidemiologic study reported that in 135 endophthalmitis cases, short-term preoperative topical antibiotics along with intracameral antibiotics didn’t reduce the rate of postoperative endophthalmitis over intracameral antibiotics alone.28 There seems to be no substantial evidence that topical antibiotics, either preoperative or postoperative, provide benefit in the prevention of endophthalmitis.
Treatments
When PCE is suspected, prompt treatment with broad-spectrum intravitreal antibiotics (vancomycin and ceftazidime, with amikacin being used for patients with a suspected penicillin/cephalosporin allergy) is critical. A benefit of vitreous tap with intraocular antibiotic injection is that it can be performed immediately in the clinic. Furthermore, the efficacy of tap and injection was reported to be non-inferior to vitrectomy by a meta-analysis of 15 retrospective case series published between January 2010 and November 2020.29 In a sample of 1,355 eyes, 55 percent received an intravitreal antimicrobial injection and 45 percent received pars plana vitrectomy. The relative risk of improvement of visual outcomes between the two groups was shown to be 1.04, indicating the viability of both treatments.29 However, surgeons in the included studies may have performed PPV on more severe cases, biasing the results.
Pars plana vitrectomy is also used as an initial treatment in certain cases as outlined by the Endophthalmitis Vitrectomy Study. The EVS is still the only large, multicenter, randomized clinical trial that investigated the visual outcomes of immediate pars plana vitrectomy versus tap and injection, both with and without systemic antibiotics.7 For eyes that had hand-motion vision or better there was no advantage to prompt vitrectomy over tap and inject alone. However, those that presented with light perception vision benefited from early vitrectomy with intravitreal antibiotics vs. just tap and inject. In this specific group, early vitrectomy resulted in a three-fold increase in the proportion of eyes recovering to 20/40 or better acuity (33 vs 11 percent), a two-fold increase of achieving 20/100 or better (56 vs 30 percent), and a 50-percent decrease in rates of severe vision loss (20 vs 47 percent) compared to those receiving primary tap and inject.7 Some smaller studies suggest that there may be a benefit to early vitrectomy. However, until larger, randomized studies are performed, the findings of the EVS still remain the standard of care.30,31
The use of intravitreal steroid injections as an adjunct treatment remains controversial. Reducing the severe inflammation typically present in acute endophthalmitis may help prevent collateral damage and could mean better visual outcomes.On the other hand, intraocular steroids could suppress the immune system’s clearance of the causative pathogen.
In any case, the currently available data don’t show definitive benefits. A prospective, randomized clinical trial indicated that out of 62 cases of endophthalmitis, the administration of intravitreal dexamethasone had no impact on visual outcomes.32 Similarly, another prospective, randomized clinical trial with 63 cases of endophthalmitis also reported no benefit from intravitreal dexamethasone.33 Thus, the use of intravitreal steroids is based on clinician preference.
In conclusion, given the high number of cataract surgeries performed in the United States each year, it’s important to remain vigilant for post-cataract surgery endophthalmitis. Several patient characteristics and intraoperative factors have been identified as potential risks for PCE. The most common causative organisms in PCE are well-described, and their detection is facilitated with traditional microbial cultures, PCR and new methodologies, including whole-genome sequencing. Both intraoperative intracameral antibiotic injection and perioperative topical povidone-iodine have been shown to be effective prophylactic interventions that reduce the risk of PCE.
Primary treatment of PCE is still guided by the recommendations of the Endophthalmitis Vitrectomy Study, with vitreous tap and injection of intravitreal antibiotics without vitrectomy as the initial treatment in all but the most severe cases.
Future studies may help to better highlight whether some eyes benefit from an earlier surgery, particularly when guided by prompt identification of the various organisms that are present in eyes with endophthalmitis.
Mr. Im is a medical student at the Sidney Kimmel Medical College at Thomas Jefferson University. Dr. Light is a vitreoretinal fellow with MidAtlantic Retina, the Retina Service of Wills Eye Hospital. Dr. Garg is a professor of ophthalmology at Thomas Jefferson University, and co-director of the Retina Research Unit at Wills Eye Hospital, where he also practices with Mid Atlantic Retina. None of the authors have a financial interest in any of the products mentioned in the article.
1. Du DT, Wagoner A, Barone SB, et al. Incidence of endophthalmitis after corneal transplant or cataract surgery in a medicare population. Ophthalmology 2014;121:1:290-298.
2. Pershing S, Lum F, Hsu S, et al. Endophthalmitis after cataract surgery in the United States: A report from the Intelligent Research in Sight registry, 2013-2017. Ophthalmology 2020;127:2:151-158.
3. Hashemian H, Mirshahi R, Khodaparast M, Jabbarvand M. Post-cataract surgery endophthalmitis: Brief literature review. J Curr Ophthalmol 2016;28:3:101-105.
4. Wisniewski SR, Capone A, Kelsey SF, Groer-Fitzgerald S, Lambert HM, Doft BH. Characteristics after cataract extraction or secondary lens implantation among patients screened for the Endophthalmitis Vitrectomy Study. Ophthalmology 2000;107:7:1274-1282.
5. Maalouf F, Abdulaal M, Hamam RN. Chronic postoperative endophthalmitis: A review of clinical characteristics, microbiology, treatment strategies, and outcomes. Int J Inflam 2012;2012:313248.
6. Rahmani S, Eliott D. Postoperative endophthalmitis: A review of risk factors, prophylaxis, incidence, microbiology, treatment, and outcomes. Seminars in Ophthalmology 2018;33:1:95-101.
7. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study Group. Arch Ophthalmol 1995;113:12:1479-1496.
8. Lalwani GA, Flynn HW, Scott IU, et al. Acute-onset endophthalmitis after clear corneal cataract surgery (1996–2005): Clinical features, causative organisms, and visual acuity outcomes. Ophthalmology 2008;115:3:473-476.
9. Fowler BJ, Miller D, Yan X, Yannuzzi NA, Flynn Jr HW. Postoperative endophthalmitis caused by Cutibacterium (formerly Propionibacterium) Acnes: Case series and review. COP 2021;12:1:1-10.
10. Lee AY, Akileswaran L, Tibbetts MD, Garg SJ, Van Gelder RN. Identification of torque teno virus in culture-negative endophthalmitis by representational deep-DNA sequencing. Ophthalmology 2015;122:3:524-530.
11. Patel SN, Storey PP, Levin H, et al. Endophthalmitis after cataract surgery: Changes in management based on microbiologic cultures. Ophthalmol Retina 2021;5:1:16-22.
12. Sjoholm-Gomez de Liano C, Soberon-Ventura VF, Salcedo-Villanueva G, et al. Sensitivity, specificity and predictive values of anterior chamber tap in cases of bacterial endophthalmitis. Eye and Vision 2017;4:1:18.
13. Seal D, Reischl U, Behr A, et al. Laboratory diagnosis of endophthalmitis: Comparison of microbiology and molecular methods in the European Society of Cataract & Refractive Surgeons multicenter study and susceptibility testing. J Cataract Refract Surg 2008;34:9:1439-1450.
14. Lee CS, Hong B, Kasi S, et al. Prognostic utility of whole genome sequencing and polymerase chain reaction tests of ocular fluids in post-procedural endophthalmitis. Am J Ophthalmol 2020;217:325-334.
15. Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology 1991;98:12:1769-1775.
16. Halachmi-Eyal O, Halachimi-Eyal O, Lang Y, Keness Y, Miron D. Preoperative topical moxifloxacin 0.5% and povidone-iodine 5.0% versus povidone-iodine 5.0% alone to reduce bacterial colonization in the conjunctival sac. J Cataract Refract Surg 2009;35:12:2109-2114.
17. Nentwich MM, Rajab M, Ta CN, et al. Application of 10% povidone iodine reduces conjunctival bacterial contamination rate in patients undergoing cataract surgery. Eur J Ophthalmol 2012;22:4:541-546.
18. Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: Results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg 2007;33:6:978-988.
19. Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg 2013;39:1:8-14.
20. Haripriya A, Chang DF, Ravindran RD. Endophthalmitis reduction with intracameral moxifloxacin prophylaxis: Analysis of 600 000 Surgeries. Ophthalmology 2017;124:6:768-775.
21. Herrinton LJ, Shorstein NH, Paschal JF, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology 2016;123:2:287-294.
22. George NK, Stewart MW. The routine use of intracameral antibiotics to prevent endophthalmitis after cataract surgery: How good is the evidence? Ophthalmol Ther 2018;7:2:233-245.
23. Arbisser LB. Safety of intracameral moxifloxacin for prophylaxis of endophthalmitis after cataract surgery. J Cataract Refract Surg 2008;34:7:1114-1120..
24. Light JG, Falkenberry SM. Unilateral bilateral acute iris transillumination-like syndrome after intracameral moxifloxacin injection for intraoperative endophthalmitis prophylaxis. JCRS Online Case Reports 2019;7:1:3-5.
25. Witkin AJ, Chang DF, Jumper JM, et al. Vancomycin-associated hemorrhagic occlusive retinal vasculitis: Clinical characteristics of 36 Eyes. Ophthalmology 2017;124:5:583-595.
26. Witkin AJ, Shah AR, Engstrom RE, et al. Postoperative hemorrhagic occlusive retinal vasculitis: Expanding the clinical spectrum and possible association with vancomycin. Ophthalmology 2015;122:7:1438-1451.
27. Carron A, Samudio M, Laspina F, et al. [Efficacy of topical 0.3% ciprofloxacin application in reducing the conjunctival biota of patients undergoing cataract extraction]. Arch Soc Esp Oftalmol 2013;88:9:345-351.
28. Friling E, Lundström M, Stenevi U, Montan P. Six-year incidence of endophthalmitis after cataract surgery: Swedish national study. J Cataract Refract Surg 2013;39:1:15-21.
29. Far PM, Yeung SC, Farimani PL, et al. TAP and inject versus pars plana vitrectomy for postprocedural endophthalmitis: A meta-analysis. RETINA 2021;41:10:2009-2016.
30. Barry P, Behrens-Baumann W, Pleyer U, Seal D. ESCRS Guidelines on prevention, investigation and management of post-operative endophthalmitis. The European Society for Cataract & Refractive Surgeons. Published online November 30, 2006.
31. Dib B, Morris RE, Oltmanns MH, Sapp MR, Glover JP, Kuhn F. Complete and early vitrectomy for endophthalmitis after cataract surgery: An alternative treatment paradigm. Clin Ophthalmol 2020;14:1945-1954.
32. Albrecht E, Richards JC, Pollock T, Cook C, Myers L. Adjunctive use of intravitreal dexamethasone in presumed bacterial endophthalmitis: A randomised trial. Br J Ophthalmol 2011;95:10:1385-1388.
33. Das T, Jalali S, Gothwal VK, Sharma S, Naduvilath TJ. Intravitreal dexamethasone in exogenous bacterial endophthalmitis: Results of a prospective randomised study. Br J Ophthalmol 1999;83:9:1050-1055.




