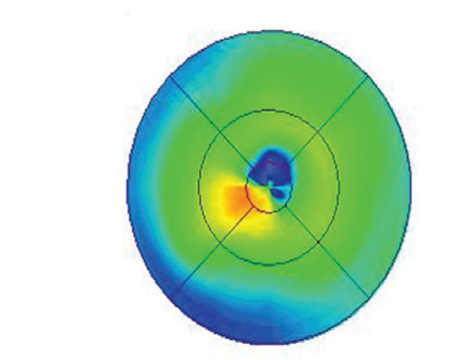
Presentation
A 61-year-old white male presented with decreased central and peripheral vision, nyctalopia and photopsias OU over the previous four months. Systemic review was significant for fatigue, difficulty sleeping, decreased appetite, dyspnea on exertion, as well as muscle aches and joint pain. He denied weight loss, fevers, chills, scalp tenderness, jaw claudication, chest pain or gastrointestinal or genitourinary symptoms.
Medical History
Past medical history was significant for obesity (BMI>41), obstructive sleep apnea on CPAP, hyperthyroidism treated with radioactive iodine and thyroidectomy, depression and migraines. His only medication was levothyroxine.
The patient was initially admitted after presenting to a general emergency room, and evaluated by several services, including neurology, cardiology, vascular surgery and ophthalmology. He had a superotemporal branch retinal artery occlusion in the right eye. Subsequent cardiac and vascular workup was overall non-diagnostic for his visual symptoms. This included an unremarkable EKG, MRI and computed tomography (CT) scan of the head and orbits, MRA/MRV, echocardiogram, carotid duplex study, BMP, CBC, LFTs, TSH, A1c and lipid panel. A stress test revealed a moderate-sized partially reversible inferior wall defect, with a left ventricular ejection fraction of 57 percent. The patient was started on a daily aspirin, but declined a statin. His erythryocyte sedimentation rate and C-reactive protein were found to be elevated at 47 and 16.5, respectively, and he was initially started on oral prednisone 60 mg daily for possible GCA. He was then referred to our retina service.
He had quit smoking five years prior after an 80-pack-year smoking history. He denied any alcohol or substance abuse. He was allergic to penicillin. Family medical history included breast cancer, diabetes and hypertension in his mother, and history of stroke in his father.
Exam
Visual acuity on presentation was 20/40 OD and 20/300 OS. Pupils were equal and reactive, with no afferent pupillary defect. IOP was 16 OD and 13 OS. Extraocular motility was full OU. Visual fields were full to confrontation OU.
The anterior exam was normal, except for 1+ nuclear sclerotic cataracts OU. Fundoscopic exam was significant for two Hollenhorst plaques versus platelet-fibrin plugs along the proximal inferotemporal arcade of the right eye, and a small choroidal nevus in the left, as well as arteriolar attenuation and mild peripheral pigmentary changes OU.
What is your diagnosis? What further workup would you pursue? The diagnosis appears below.
Work-up, Diagnosis and Treatment
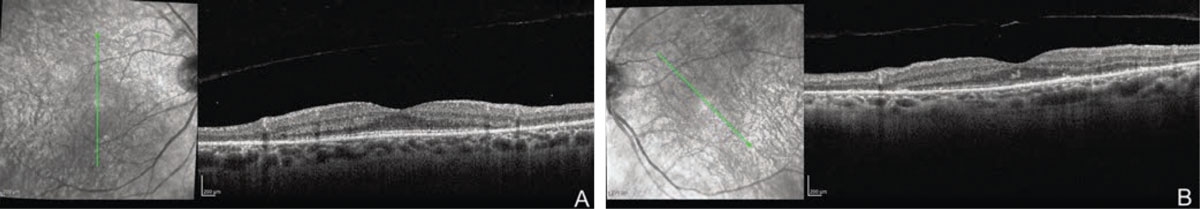
OCT demonstrated disruption of the extrafoveal ellipsoid zones in both eyes (Figure 1 A, B). No cystoid spaces or subretinal fluid were seen. Fundus autofluorescence and fluorescein angiography were within normal limits. Full-field ERG demonstrated near isoelectric responses to scotopic stimuli and combined flash stimuli OU, as well as a severe decrease in amplitude in response to single flash photopic and 30-hertz flicker stimuli, suggesting advanced retinal dysfunction in both eyes. Octopus perimetry demonstrated a superotemporal arcuate scotoma, denser temporally, as well as an inferior arcuate scotoma.
 |
|
Figure 1. OCT demonstrates disruption of the extrafoveal ellipsoid zones in the right (A) and left (B) eyes. |
Given the patient’s symptoms and workup, autoimmune retinopathies (AIR), including paraneoplastic (pAIR) and non-paraneoplastic (npAIR) etiologies, were at the top of the differential. Acute zonal occult outer retinopathy, hereditary retinal degenerations such as retinitis pigmentosa, cone-rod dystrophy, and toxic-nutritional retinopathies were considered, but thought to be less likely.
The patient reported mild improvement in symptoms after initial treatment with prednisone. However, he was unable to taper below a daily dose of 20 mg of prednisone without developing worsening visual symptoms; he was subsequently started on a steroid-sparing regimen of mycophenolate and tacrolimus. He later developed hemoptysis and worsening SOB, and was found to have a large left lung mass on CT that was then biopsied and showed poorly differentiated carcinoma with focal squamous differentiation. A PET scan showed diffuse metastases involving the liver, adrenal gland, spine, pelvic bones, femurs and multiple ribs. The patient began chemotherapy and palliative radiation for stage IVB non-small cell lung cancer, but sadly did not survive his diagnosis.
Discussion
Autoimmune retinopathies comprise a spectrum of retinal degenerative disorders characterized by subacute vision loss, visual field deficits and the presence of circulating antiretinal autoantibodies (ARAs). Depending on the presence or absence of concomitant malignancy, AIR can be broadly classified as paraneoplastic or non-paraneoplastic (npAIR). Paraneoplastic AIR can be further subdivided into cancer-associated retinopathy (CAR) and melanoma-associated retinopathy (MAR).1 Patient symptoms may reflect dysfunction of the associated photoreceptors and the damaged retinal tissue. CAR classically affects both rods and cones, whereas MAR is characterized by rod dysfunction secondary to antibodies directed toward bipolar cells. Cone dysfunction can lead to reduced visual acuity and central vision, hemeralopia and decreased color discrimination, while patients with rod dysfunction may experience more peripheral field loss, nyctalopia and prolonged dark adaptation.2,3
Cancer-associated retinopathy is most often a bilateral disease that can lead to rapid progressive visual deterioration. The differential diagnosis includes MAR, nPAIR, retinal degenerative disorders such as retinitis pigmentosa and cone-rod dystrophy, white-dot syndromes such as acute zonal occult outer retinopathy (AZOOR), toxic-nutritional retinopathy and non-infectious and infectious posterior uveitis. Currently, the diagnosis is made based on clinical presentation, abnormalities on ERG, and the presence of serum ARAs in the absence or presence of malignancy.
The fundus can initially be normal in appearance in CAR, with later stages revealing potential arteriolar attenuation, retinal pigment epithelial changes and pallor of the optic disc.4 Abnormalities of cone and rod dysfunction may be evident on ERG. Optical coherence tomography can demonstrate loss of outer retinal structures, including the ellipsoid and interdigitation zone, or show cystic spaces or occasionally mild schisis-like changes.5
Antibodies to recoverin, a retina-specific calcium-binding protein in photoreceptors, is most commonly associated with CAR.6 However, the diagnosis of autoimmune retinopathy remains challenging, in part because the presence of ARAs alone is not diagnostic, as well as lack of an accepted gold standard for ARA detection. Authors of one study sent blood specimens from 14 patients with AIR to two different labs and found the concordance rate to be only 36 percent for specimens sent from the same patients.7 ARAs can be found in other systemic autoimmune diseases, RP and AMD, as well as in retinal degenerations, uveitis and normal eyes.8,9 A recently published study in JAMA reported the presence of retinal antibodies in 93 percent of the patients without autoimmune retinopathy.10 In addition, some AIR cases have been reported in which no ARAs were found.11 While testing for serum ARAs wasn’t ultimately done for our patient, the combined clinical presentation, ancillary testing and subsequent detection of small-cell lung cancer strongly implicated CAR.
An extensive investigation, in partnership with an internist or primary care physician, should be conducted in order to determine a patient’s individual risk factors and determine age- and gender-appropriate cancer testing. CAR is most frequently associated with small-cell lung cancer, breast cancer and other gynecologic cancers.12 In most cases of CAR, vision loss precedes the diagnosis of malignancy; one such interval was reported as much as 11 years prior to diagnosis.13 In contrast, vision loss in MAR is often accompanied by a recurrence or metastasis of a previously diagnosed cutaneous melanoma.14
There are currently no clear prognostic indicators or standard parameters to guide treatment in CAR. A number of largely anecdotal case reports and observational studies have reported on different treatments for CAR, with variable results and improvement in visual function. These treatment modalities include regional corticosteroid injections, systematic immunosuppressive medications (cyclosporine, azathioprine, alemtuzumab, rituximab and intravenous immunoglobulin) and plasmapheresis.15-18 In any case, prognosis remains poor once widespread retinal degeneration occurs.
1. Heckenlively JR, Ferreyra HA. Autoimmune retinopathy: A review and summary. Semin Immunopathol 2008;30:2:127–134.
2. Chan JW. Paraneoplastic retinopathies and optic neuropathies. Survey of Ophthalmology 2003 48:1:12-38.
3. Raj MK, Purvin VA. Cancer associated and related autoimmune retinopathy. eMedicine. http://emedicine.medscape.com/article/1227724-overview.
4. Jacobson DM, Thirkill CE, Tipping SJ. A clinical triad to diagnose paraneoplastic retinopathy. Ann Neurol 1990;28:162–167.
5. Grewal DS, Fishman GA, Jampol LM. Autoimmune retinopathy and antiretinal antibodies: A review. Retina 2014;34:827–845.
6. Polans AS, Witkowska D, Haley TL, et al. Recoverin, a photoreceptor-specific calcium-binding protein, is expressed by the tumor of a patient with cancer-associated retinopathy. Proc Natl Acad Sci USA 1995;92:20:9176–9180.
7. Faez S, Loewenstein J, Sobrin L. Concordance of antiretinal antibody testing results between laboratories in autoimmune retinopathy. JAMA Ophthalmol 2013;131:1:113–115.
8. Hooks JJ, Tso MO, Detrick B. Retinopathies associated with antiretinal antibodies. Clin Diagn Lab Immunol 2001;8:5:853-58.
9. Dutta Majumder P, Marchese A, Pichi F, Garg I, Agarwal A. An update on autoimmune retinopathy. Indian J Ophthalmol 2020;68:9:1829-1837.
10. Chen JJ, McKeon A, Greenwood TM, et al. Clinical utility of antiretinal antibody testing. JAMA Ophthalmol April 22, 2021. doi:10.1001/jamaophthalmol.2021.0651. [epub ahead of print]
11. Grange L, Dalal M, Nussenblatt RB, et al. Autoimmune retinopathy. Am J Ophthalmol 2014;157:2:266-272.
12. Dot C, Guigay J, Adamus G. Anti-alpha-enolase antibodies in cancer-associated retinopathy with small cell carcinoma of the lung. Am J Ophthalmol 2005;139:746–7.
13. Saito W, Kase S, Ohguro H, Furudate N, Ohno S. Slowly progressive cancer-associated retinopathy. Arch Ophthalmol 2007;125:10:1431-3.
14. Keltner JL, Thirkill CE, Yip PT. Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: Eleven new cases and a review of 51 previously published cases. J Neuroophthalmol 2001;3:173-87.
15. Keltner JL, Thirkill CE, Tyler NK, Roth AM. Management and monitoring of cancer-associated retinopathy. Arch Ophthalmol 1992;110:48–53.
16. Ferreyra HA, Jayasundera T, Khan NW, He S, Lu Y, Heckenlively JR. Management of autoimmune retinopathies with immunosuppression. Arch Ophthalmol 2009;127:4:390–397.
17. Espandar L, O’Brien S, Thirkill C, Lubecki LA, Esmaeli B. Successful treatment of cancer-associated retinopathy with alemtuzumab. J Neurooncol 2007;83:295–302.
18. Davoudi S, Ebrahimiadib N, Yasa C, Sevgi DD, Roohipoor R, Papavasilieou E, et al. Outcomes in autoimmune retinopathy patients treated with rituximab. Am J Ophthalmol 2017;180:124–32.