There’s an old adage that the more treatments there are for a given disease or condition, the less likely it is that any of them is ideal. No refractive vision condition exemplifies this more than presbyopia, which has had every treatment under the sun thrown at it over the years, but a perfect solution has still remained elusive. One new approach that’s close to arrival in the United States is the corneal inlay, which proponents say offers the benefit of an effective yet removable option for presbyopes. Here’s an update on three inlays and their current results.
Acufocus Kamra
According to John Vukich, MD, who was an investigator in the Kamra’s U.S. trial, the Kamra inlay is far along in the Food and Drug Administration approval process, and 30,000 of them have already been implanted in patients outside the United States. The implant is designed to be used in emmetropic presbyopes.
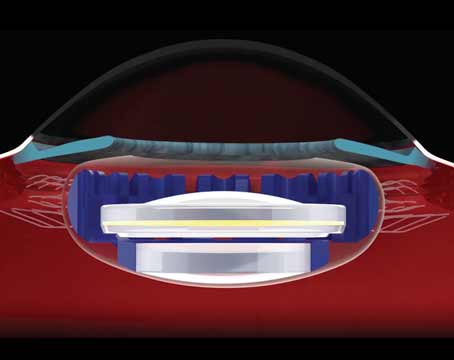
“The Kamra is implanted monocularly, usually in the non-dominant eye,” Dr. Vukich explains. “It works by essentially creating a pinhole effect with its central 1.6 mm aperture. The outer diameter of the ring is 3.2 mm. It has no refractive power, and it doesn’t alter the refractive power or the shape of the cornea. It doesn’t require power selection or an adjustment for age. A patient won’t outgrow it to the point where you would need to exchange it for an inlay of another power. It’s a reduced aperture size that, by using the central collimated light rays, is able to create a depth of focus that allows near vision restoration.”
For implantation, the surgeon uses a femtosecond laser to create a stromal pocket at 180 to 200 µm depth. The surgeon uses a special modified forceps to slide the inlay into the pocket. “We’ve learned that the quality of the stromal bed smoothness and the quality of the incision have a significant impact on the outcome,” says Dr. Vukich. “Because these are placed at 180 to 200 µm, the laser profile needs to be adjusted carefully. The tighter the laser’s spot/line separation—in other words, the closer the spots are to each other and the closer a line of spots is to each adjacent line—the smoother the cut is. At deeper levels this smoothness makes a significant difference in terms of the quality of vision. So, some of the earliest patients in whom we didn’t understand or recognize this correlation didn’t get as good of an effect for near vision as we had hoped, and some of their implants were removed because they got limited benefit from them. Later in the trial, once we had established a connection between the quality of the stromal bed, the spot/line separation and the visual quality outcomes, patients had much better results.”
In the U.S. study of the Kamra, 428 patients with two years of follow-up gained 3.2 lines of near acuity on average, and are reading at approximately J2. Intermediate vision in the inlay eye improved from less than 20/32 to 20/25. The mean uncorrected distance vision in the inlay eye is 20/20. “There haven’t been any major complications,” Dr. Vukich says. “Some inlays were removed for a variety of reasons, but this is a small number, less than a fraction of a percent. A common reason for removal was related to centration and positioning, but we’ve certainly learned a lot about how to position and appropriately place them now.”
Dr. Vukich says that, in terms of FDA approval, the company is collating the final three-year data on the trial patients and is putting it all together for an FDA submission that could occur very soon.
Presbia Flexivue Microlens
Rather than use a small-aperture pinhole effect, the Flexivue is a thin ring that contains a refractive power that alters the eye’s index of refraction, conferring some near vision ability to the patient.
“The power that’s selected depends on the patient,” explains Bogota, Colombia, ophthalmologist Gustavo Tamayo, a medical advisor to Presbia. “We can go from +1 to +3 D, depending on the age and needs of the patient, and his previous refraction. The inlay is implanted in the non-dominant eye, so we’re not decreasing the patient’s binocular distance vision because the dominant eye, which is assumed to be 20/20 for distance, remains untouched.” The inlay is 3 mm in diameter, with a 0.9-mm central hole, and is 15 µm thick. It’s intended to be implanted in emmetropic presbyopes. The company advises that patients undergo a monovision test of +1.5 D beforehand. “It’s not ‘real’ monovision,” says Dr. Tamayo. “It’s one eye seeing 20/30 and the other 20/20. However, you have to produce a little in the patient and see if he can tolerate that small difference.”
“We use a femtosecond laser to create a pocket at a depth of 300 µm,” Dr. Tamayo continues. “We then insert the inlay in the pocket. The pocket could be created by any instrument, such as a modified microkeratome, but the company requires a femtosecond laser in its protocol. Centering the implant is a very big issue right now for any type of correction of presbyopia, because we don’t know exactly where a patient’s visual axis is. We advise surgeons to center the inlay on the second Purkinje reflex, which is usually closer to the visual axis than the center of the pupil. In fact, for a surgeon’s first cases, I recommend that he have a slit lamp close to the surgical theater in order to view the eye postop to make sure the inlay’s perfectly centered. However, an advantage of this inlay is that you can center it the next day after implantation without any problem if you notice it’s not centered.”
In a paper presented at the 2012 meeting of the European Society of Cataract and Refractive surgery by Vladimir Feingold, Ioannis Pallikaris, MD, and Marco Fantozzi, MD, 75 percent of 54 emmetropes implanted with the Flexivue could read J2 or better at 12 months. The uncorrected distance vision in the operated eye decreased from 20/20 to 20/40, but best-corrected vision remained 20/20. Dr. Tamayo says that, in some cases, an inlay of one power needs to be exchanged for a stronger one as the patient ages. “We have done this when patients become more presbyopic,” he says. “The ones who are used to the difference between the eyes can tolerate the change.”
In terms of adverse events, some lenses have to be exchanged from time to time. “In inexperienced surgeons who aren’t used to dealing with presbyopia surgery, the exchange rate can be as high as 30 percent,” says Dr. Tamayo. “But for experienced surgeons, the removal rate is between zero and 1 percent, because they have already tested the patients, and the patients know what to expect. I’ve had no reason to explant an inlay except for one case in which the patient developed a vitreous hemorrhage months after surgery for a reason unrelated to the inlay. Once you gain experience with the surgery, the repositioning rate is almost zero, as well, and I’d say it’s no greater than 5 percent. For the inexperienced surgeon, the need to come back and recenter an inlay may be between 10 and 20 percent.” About half of the patients get halos that last for about a month postop, Dr. Tamayo adds.
It could be a couple of years before the Flexivue is ready for FDA evaluation. “It’s not approved in the United States,” says Dr. Tamayo. “We’re just starting the FDA study of the inlay around the time of this year’s ASCRS. Having one of the inlay designs that completes all the studies in the United States will take one or two years.”
ReVision Optics Raindrop
A third proposed mechanism for treating presbyopia is being pursued by the Raindrop inlay: Rather than using the pinhole effect or containing an add power, the Raindrop relies on using its own thickness to create a small bump that alters the cornea’s curvature in the emmetropic presbyope’s non-dominant eye.
“The Raindrop is a 2-mm-diameter, 30-µm-thick device that is implanted under a corneal flap that’s a little thicker than that normally used for LASIK—150 µm,” explains John Olkowski, MD, a Honolulu surgeon who consults for ReVision Optics. “The presence of the implant causes a central elevation in the flap that generates multifocality in the cornea. By not having a refractive power, if the device settles a little off center, off the visual axis, it won’t impact visual results. This attribute gives the surgeon a little bit of a safety net versus a device where, if it’s a little off-center, the patient becomes symptomatic.” Dr. Olkowski says that the operated eye will lose about a line of distance vision, but that this isn’t noticeable binocularly. Also, a small percentage of patients will develop some corneal haze around the device, but an additional course of steroids can treat this successfully.
To help ensure success with the device, surgeons in the Raindrop FDA trial give prospective patients a multifocality test before implantation. “We have to do a multifocal contact lens trial on the non-dominant eye to see if they can tolerate that,” Dr. Olkowski says. “They wear the lens for five days to see what they think. This is pretty extreme—if they can tolerate that lens for five days, they’ll most likely love the inlay, because the contact lens can slide around and has its own challenges.”
The Raindrop is poised to enter the final years of its FDA trial. “We received approval for the last phase of our study, Phase IIIb, this March and are recruiting for it now,” Dr. Olkowski says. “We’ll be enrolling 300 patients in this Phase IIIb study.”
REVIEW