Here, I’d like to review some of what we’ve learned about mitomycin-C over the past 25 years, to help ensure that we use this drug appropriately and safely. I’ll also note some of the differences between mitomycin and 5-fluorouracil, another drug in wide use for similar purposes.
Mitomycin-C: An Overview
Mitomycin-C is an alkylating, anti-tumor antibiotic, generally used in surgery because of its ability to inhibit fibroblast proliferation and suppress vascular ingrowth—two parts of the healing process that can undercut the beneficial effect of a trabeculectomy bleb by producing scarring that stops aqueous outflow. One of mitomycin’s active metabolites cross-links with DNA, causing selective interruption of DNA replication, thus inhibiting mitosis and protein synthesis. This makes mitomycin cytotoxic for both fibroblasts and microvascular endothelial cells, so it not only reduces the production of fibroblasts, but also the microvascular blood supply to the area treated.
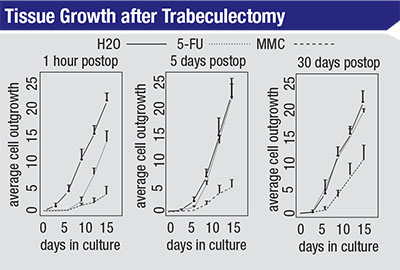 |
| The growth rate of scleral fibroblast cells in culture after treatment with H2O, 5-FU or MMC in a rabbit model. Cells were taken from the treated area at the times indicated and then cultured. In each case, MMC-treated cells grew more slowly than those treated with 5-FU after 15 days in culture. Cells treated with 5-FU that were cultured five or 30 days after treatment exhibited growth curves similar to cells treated with water. (Khaw PT, Doyle JW, et al, 1993)1 |
One reason mitomycin is so ef-fective is that it’s not dependent on the cell-cycle phase. In contrast, 5-FU is only effective at certain phases of the cell’s life cycle. You can see the results of this difference in studies that compare the effectiveness of the two agents. For example, in a study done in 1993, researchers took cells from eyes that had been treated with distilled H2O, 5-FU or mitomycin and then grew them in culture to reveal their relative state of health.1 (See charts, top of page 104.) They took the cells after different periods of time postoperatively—at one hour, five days and 30 days postop. The cells were then cultured for 15 days.
The left-hand graph shows the relative impact on cell growth when the cells were taken from the treated area one hour after surgery. The cells treated with water show a fairly exponential growth rate over the following 15-day period. The cells that were exposed to 5-FU show delayed growth for seven to nine days, but then they resume growing at about the same rate as those treated with water. The third line in the graph shows that when treated with mitomycin, even after 15 days the cells’ growth rate is still very slow compared to the other two treatments.
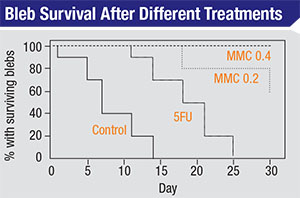 |
| Blebs treated with water, 5-FU and two different strengths of mitomycin-C (in a rabbit model) show dramatically different survival rates. (Khaw PT, Doyle JW, et al, 1993)2 |
The bottom line of this experiment is that you can almost think of the 5-FU as having a temporary effect on the fibroblasts. After that temporary effect—lasting five to seven days— wears off, the cells pretty much resume growing as if they had not been treated. In contrast, mitomycin has a long-term effect on the fibroblasts; even 30 days after treatment the cells’ growth rate is still reduced.
Another 1993 study compared bleb survival rates following the application of water, 5-FU and two different strengths of mitomycin over a 30-day postoperative period in a rabbit model.2 (See graph below.) The control blebs (treated with water) were almost all gone by day 14; the ones treated with topically applied 5-FU lasted until day 25; about 60 percent of the blebs treated with mitomycin 0.2 survived until day 30; and virtually all of the blebs treated with mitomycin 0.4 survived to day 30. (One takeaway from this is that we have the ability to titrate this effect; we don’t have to choose between no effect and maximum effect—with all of the potential side effects potentially accompanying the latter.)
Given these data, it’s reasonable to ask why anyone would still use 5-FU. For one thing, 5-FU produces good results in many patients. If you can control the scarring response early on and get a good bleb established, you often can go on to get a very good long-term result. 5-FU can accomplish that in many patients. In addition, two studies comparing the use of mitomycin versus 5-FU in normal-tension glaucoma patients found that outcomes were often better when 5-FU was used instead of mitomycin.3,4
Minimizing Toxicity
Mitomycin is clearly effective at prolonging the life of a bleb, but it creates this effect by interfering with DNA replication, so it’s not hard to imagine how this can backfire. The side effects of treatment with mitomycin were well-illustrated by a 1991 study involving four New Zealand white rabbits.5 In this study, 50 µl of 0.5 mg/ml mitomycin—a fairly strong formulation—was injected into the anterior chamber. Within 24 hours the eyes showed severe inflammation; within 72 hours the re-searchers found corneal opacification. By two weeks post-injection, the iris and ciliary body were necrotic and the cornea was distressed; the endothelium was basically gone. Clearly, full-strength mitomycin in the anterior chamber is highly toxic.
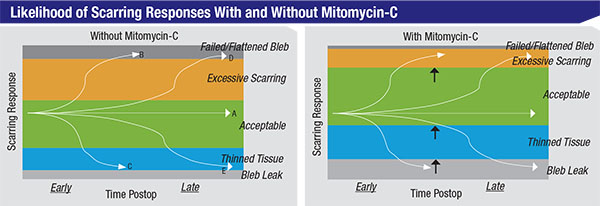 |
| Using mitomycin-C alters the likelihood of different outcomes following trabeculectomy. The green zones represent acceptable capsule thickness; the orange zones represent excessive scarring, leaving the capsule too thick and pressure higher than desired; the upper dark grey zones, blebs that have scarred over completely and failed; the blue zones, blebs with thin tissue and possibly too low a pressure or leakage; and the lower light grey zones, leaky, thin, avascular blebs. The curving lines running across the charts represent different patients. Even without MMC, 60 or 70 percent of trabeculectomy patients will do beautifully (like patient A). Some, like patient B, will get excessive scarring and a failed bleb early on, while others, like patient D, will get that result sometime later. Some patients, like patient C, will develop thinning tissue early on, while others, like patient E, will have this outcome late in the postoperative course—possibly even years after the surgery. The differences between the left and right charts show how the likelihood of different outcomes shifts when mitomycin-C is used. Using mitomycin shifts the likely scarring response away from excessive scarring and towards thinned tissues. |
Other studies have demonstrated similar damaging effects:
• One study reported limbal stem cell deficiency after subconjunctival injection of 0.1 to 0.2 ml of 0.2 mg/ml mitomycin, a delivery method that more and more surgeons are using.6
• Another study found greater corneal endothelial cell loss at three months in patients getting trabeculectomies with mitomycin than in those undergoing trabeculectomy without mitomycin.7
• A third study using a relatively low 0.1 to 0.2 mg/ml dose to treat the intact sclera in a rabbit model found pathologic changes in the ciliary epithelium, nerves within the ciliary body and the ciliary body capillaries even six to 12 months postoperatively.8
In clinical practice, we rarely see problems that are clearly connected to the use of mitomycin. However, as these studies demonstrate, mitomycin may cause limbal cell deficiency, corneal endothelial cell loss, changes in the ciliary body and reduced aqueous production.
Obviously, mitomycin-C is not something we should be using carelessly. Fortunately, we have a number of ways to titrate the impact of the drug. We can control:
• the concentration we use;
• the method of application (e.g., via sponge or injection);
• the duration of application;
• the surface area treated; and
• the amount of rinsing we do after the application is complete.
A study published in 1997 demonstrated the impact of the amount of surface area treated with mitomycin.9 The study involved 24 New Zealand white rabbits; one-third of them were treated over a large 8x10-mm surface area, one-third over a smaller 4x2-mm area, and a no-treatment group acted as a control. At 21 days researchers found that the large treatment area led to improved bleb survival (p<0.039), larger bleb area (p<0.009), better bleb height (p<0.005) and a more diffuse bleb that wasn’t as thin-walled or localized as that seen in the other two groups.
The reason for wanting to create a large, diffuse bleb—which may sound counterintuitive to the uninitiated—is that a small, localized bleb tends to generate a “ring of steel” around the area you’ve treated. This leads to thinning of the tissues that you’ve treated, because the aqueous has nowhere to go beyond that localized area. To avoid this, you want to create a wide, diffuse bleb with a large surface area, perhaps using a lower concentration and shorter duration to limit toxicity.
The amount of irrigation we apply has also been shown to affect how much mitomycin makes it into the anterior chamber, which (as already noted) can have negative effects. In one 1995 study, researchers put a 6x4-mm cellulose sponge, soaked in mitomycin 0.5 mg/ml, on the eyes of a rabbit for five minutes.10 Then, one eye was irrigated with 10 ml of saline for one minute; the other eye was left without irrigation. Then they looked at the aqueous levels of mitomycin over the course of an hour. The eyes which received irrigation to wash off the mitomycin had much lower mitomycin concentrations at all time points, and a continuous drop in concentration after 15 minutes. The eye not irrigated showed a continuous rise in aqueous mitomycin level over the course of the hour.
Given all of these factors that are within our control, it would be convenient to have a formula to guide us, in terms of which concentration to use, how large an area to cover, how long to apply it and how long to rinse the eye after application. Unfortunately, such a formula doesn’t exist, because different eyes react differently to the same use of mitomycin (in the same surgery performed by the same surgeon). The results of seemingly identical treatment can range from a thick, encapsulated bleb to a nice diffuse, not-too-thin bleb to a very thin, avascular bleb. In fact, our inability to predict how a given patient will respond to mitomycin undoubtedly accounts—at least in part—for the less-than-100-percent rate of excellent outcomes associated with trabeculectomy.
We do know a few things. We know that younger, fitter patients heal better than older, less-well patients. We know that patients of African ancestry tend to scar more than Caucasian patients. We know that patients with one eye that develops an encapsulated bleb have more risk of developing one in the other eye. But on the whole, we’re partly guessing; we really don’t know how a patient is going to respond to a trabeculectomy surgery. The best we can do is make an educated guess, based on our experience and the limited data that’s available.
The Impact of MMC
As surgeons, we tend to think of mitomycin-C as a positive agent, which in general it is. However, it’s not a cure-all. What it does is shift the likelihood of certain types of outcomes in a direction that is more favorable to our patients’ long-term vision and comfort (as illustrated in the charts at the top of page 106). Essentially, by using mitomycin, we’ve shifted the likely response away from excessive scarring and towards thinned tissues.
Of course, these responses—too much or little scarring—will sometimes occur whether we use mitomycin or not. Mitomycin simply changes the relative postoperative risks toward less risk of excessive scarring and a failed bleb, and greater risk of thin tissues and bleb leaks. Again, mitomycin is not a cure-all; you have to pick your poison.
My intention here is not to minimize the value of mitomycin. We do tend to get better outcomes when we use it than when we don’t, and there’s no question that reducing excessive scarring is a significant change. But it’s easy to focus on the potential complications that can result from the use of mitomycin. We all think about the potential for bleb leaks, endophthalmitis and blebitis. There is reason for concern, but it’s also important to keep your perspective.
Consider a study published in 1997 that compared the number of patients developing endophthalmitis each year at the Bascom Palmer Eye Institute, divided into two groups based on whether or not mitomycin was used during the surgery.11 From 1989 to 1991 there was no mitomycin use; on average, three patients per year developed endophthalmitis. Then, as mitomycin became part of some surgeries in ‘94 and ‘95, the number jumped. In 1995 in particular, the group receiving mitomycin had a significantly higher number of cases of endophthalmitis than the nonmitomycin group—five, compared to one. This clearly suggests that using mitomycin may lead to more endophthalmitis. On the other hand, we’re talking about one to six cases of endophthalmitis per year out of a very large number of patients undergoing trabeculectomy surgery at Bascom Palmer during this period. Furthermore, these numbers only present one side of the story; they don’t tell us how many blebs would have failed, but didn’t because of the mitomycin.
The reality is, use of mitomycin dramatically increases the number of blebs that continue to function for many years—blebs that may not have continued to work if we hadn’t used mitomycin. That’s the reason we use it. If we get an extra 15 patients to do well long-term out of every 100, adding one extra patient with a problem out of that 100 might be considered to be a reasonable trade-off, especially since glaucoma is potentially vision-threatening if not adequately controlled. Remember: People with glaucoma most commonly go blind because of a lack of pressure control—not because of the complications of surgery.
Making the Best of It
Back in the early 1990s, many surgeons were reluctant to use mitomycin. Today, however, very few glaucoma surgeons fail to use mitomycin on the vast majority of their surgical patients. Mitomycin is a very useful and effective tool, but it’s not a perfect solution to the problem of scarring. It’s very effective at decreasing fibrosis and improving bleb survival, but it increases bleb thinning and side effects, especially long-term. Sometimes it’s too effective; sometimes it’s not effective enough. It’s a potentially toxic agent, and individual variations make the results of using it unpredictable. We get better at using it as a result of experience, but we have to recognize that the outcome for any given patient is beyond our ability to predict.
A few basic tenets for its use have held up. Because the effects are localized to the area treated, it’s important to treat a large surface area to decrease the risk of a “ring of steel” forming, with a thin ischemic anterior bleb. Rinsing the eye thoroughly with BSS is helpful in minimizing the damaging effects described earlier. Some surgeons use more intense mitomycin treatment on younger patients because of their superior healing, but it’s important to remember that the younger the patient, the longer the bleb has to last and the greater the risk that, over time, a thin bleb will allow an infection to get inside the eye. For example, if the infection rate is half a percent per year, a 90-year-old patient who survives five years has a 2.5 percent total risk. But if the patient is 30 and may live another 50, 60 or 70 years, that half-percent-per-year risk becomes much more significant. So you always have to weigh the effectiveness of your treatment against the possible risks.
People have been trying to develop alternative surgeries that don’t require a bleb, such as the minimally invasive glaucoma surgeries, but as everyone knows, the weakness of those procedures is their less-dramatic reduction in pressure. At the same time, people have spent many years trying to develop better agents for getting an antifibrosis effect when creating a bleb, but they haven’t found any-thing else that works as well as mitomycin—at least so far. That’s the conundrum: Even though there are risks associated with using mitomycin, the risk of losing vision is far greater if our surgery fails to lower the patient’s pressure over the long run. REVIEW
Dr. Sherwood is a professor of ophthalmology and director of the Center for Vision Research at the University of Florida. He is a past recipient of the American Academy of Ophthalmology’s Honor Award.
1. Khaw PT, Doyle JW, Sherwood MB, Grierson I, Schultz G & McGorray S. Prolonged localized tissue effects from 5-minute exposures to fluorouracil and mitomycin C. Arch Ophthalmol 1993;111:2:263-7.
2. Khaw PT, Doyle JW, Sherwood MB, Smith MF, McGorray S. Effects of intraoperative 5-fluorouracil or mitomycin C on glaucoma filtration surgery in the rabbit. Ophthalmology 1993;100:3:367-72.
3. Membrey WL, Poinoosawmy DP, Bunce C, Hitchings RA. Glaucoma surgery with or without adjunctive antiproliferatives in normal tension glaucoma: 1: Intraocular pressure control and complications. Br J Ophthalmol 2000;84:6:586-90.
4. Membrey WL, Bunce C, Poinoosawmy DP, Fitzke FW, Hitchings RA. Glaucoma surgery with or without adjunctive antiproliferatives in normal tension glaucoma: 2: Visual field progression. Br J Ophthalmol 2001;85:6:696-701.
5. Derick RJ, Pasquale L, Quigley HA, Jampel H. Potential toxicity of mitomycin C. Arch Ophthalmol 1991;109:12:1635.
6. Sauder G, Jonas JB. Limbal stem cell deficiency after subconjunctival mitomycin C injection for trabeculectomy. Am J Ophthalmol 2006;141:6:1129-30.
7. Sihota R, Sharma T, Agarwal HC. Intraoperative mitomycin C and the corneal endothelium. Acta Ophthalmol Scand 1998;76:1:80-2.
8. Mietz H, Addicks K, Bloch W, Krieglstein GK. Long-term intraocular toxic effects of topical mitomycin C in rabbits. J Glaucoma 1996;5:5:325-33.
9. Cordeiro MF, Constable PH, Alexander RA, Bhattacharya SS, Khaw PT. Effect of varying the mitomycin-C treatment area in glaucoma filtration surgery in the rabbit. Invest Ophthalmol Vis Sci 1997;38:8:1639-46.
10. Prata JA Jr, Minckler DS, Koda RT. Effects of external irriga-tion on mitomycin-C concentration in rabbit aqueous and vitreous humor. J Glaucoma 1995;4:1:32-5.
11. Kangas TA, Greenfield DS, Flynn HW Jr, Parrish RK 2nd, Palmberg P. Delayed-onset endophthalmitis associated with conjunctival filtering blebs. Ophthalmology 1997;104:5:746-52.



