Proliferative vitreoretinopathy is an aberrant vitreoretinal wound-healing process that leads to formation of proliferative contractile membranes following primary rhegmatogenous retinal detachment. PVR has been reported to occur in approximately 10 percent of RRD cases, with a higher incidence in cases with risk factors, and is the leading cause of RRD surgery failure.1,2 PVR often leads to recurrent retinal detachments that are complicated in nature with a tractional component, require additional surgery, and have a guarded visual prognosis. Here, we’ll provide advice on the best way to manage these cases surgically.
Pathophysiology
Understanding the development of PVR is helpful in preventing and managing it. Inflammation is an important initial step in PVR. Retinal detachment leads to ischemia and cell death, which results in breakdown of the blood-retinal barrier. This allows for an influx of cytokines, growth factors, blood-borne immune cells and other blood components.3 Cytokines induce migration and proliferation of the resident retinal pigment epithelial cells, a major cell type involved in the pathogenesis of PVR. The RPE cells adhere to the retina, undergo transformation into mesenchymal cells and form proliferative fibrocellular membranes which acquire contractile abilities that can lead to complex rhegmatogenous and tractional retinal detachments.4,5
Risk Factors
Nearly all risk factors for PVR formation are related to liberation of retinal pigment epithelial cells and breakdown of the blood-retinal barrier.6-8 Risk factors for postoperative PVR are related to the number and size of retinal breaks, extent of the retinal detachment and the presence of inflammation or PVR preoperatively.9,10 Other identified risk factors include trauma—especially penetrating or perforating—giant retinal tears, prolonged inflammation of the posterior segment, viral infections of the posterior segment, prolonged chorioretinitis, vitreous hemorrhage and multiple previous surgeries. Additional risk factors include RDs with choroidal detachments and RDs associated with genetic syndromes. The incidence of postoperative PVR is higher in children and pediatric PVR is usually characterized by an aggressive course.11
 |
Prevention
Presently there is no pharmacologic treatment proven to prevent PVR formation in its entirety. Current strategies for PVR prevention are focused on timely and successful repair of RRD. Care should be taken to avoid iatrogenic breaks in eyes with inflammation, endophthalmitis, chorioretinitis, and in pediatric RD, where the risk of PVR is increased.
Postoperatively, consider closely following patients who are at increased risk. The highest-risk period for PVR formation is four to 12 weeks following primary RD repair.
Features and Classification
Clinically, early stages of PVR are characterized by cellular dispersion in the vitreous and white opacification of the retinal surface with increased reflectance and a cellophane-like appearance. Retinal tears can have rolled edges, retinal folds can have fine membranes between them, and the mobility of the detached retina is reduced. The hallmark feature of PVR is formation of preretinal membranes, resulting in retinal wrinkling and characteristic fixed star-fold formation. In severe cases of PVR, narrow or closed funnel detachments form.
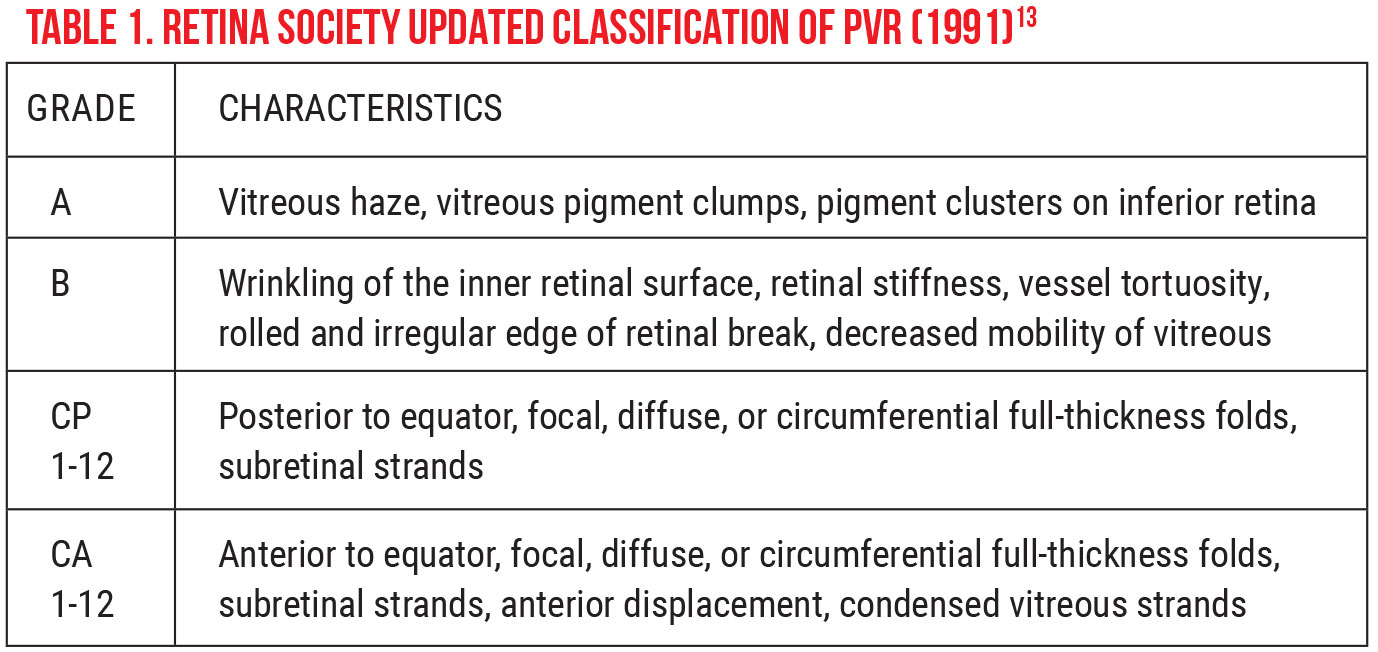
The most popular classification scheme remains that which was published by the Retina Society Terminology Committee in 1983. In this classification, PVR is subdivided into four stages of increasing severity, A to D, that run from minimal to massive.12 (Some limitations of this classification have been identified, including lack of specified location and magnitude of traction.) In 1991, the updated Retina Society Classification eliminated grade D PVR and expanded on grade C, emphasizing the posterior vs. anterior locations of proliferation, added various types of contraction and added the ability to note the extent of the disease in clock hours (See Table 1 on p.78).13 This new classification is detailed and, while it’s somewhat difficult to incorporate into clinical practice, it provides standardized nomenclature that’s useful for clinical trials.14 Even so, PVR classification is a useful tool for grading severity, discussing patient expectations, communicating with colleagues and performing research.
PVR is a clinical diagnosis. Occasionally, in cases of media opacity limiting a view to the fundus, ancillary imaging, such as ultrasonography, is necessary. Dynamic ultrasonography is especially helpful in this scenario and reveals less retinal mobility during the exam in patients with PVR-related RD, due to fixed retinal folds.
Management
Given the lack of effective medical therapy, surgery is the main treatment for PVR. Timing of PVR surgery depends on the case. Some authors advocate delaying surgery in early cases of postoperative PVR out of a concern for heightening the inflammatory response and stimulating further PVR formation if additional procedures are performed.15-17 Furthermore, delaying repair leads to the formation of mature membranes, which are generally easier to remove than fragile, immature membranes that are difficult to remove in sheets.17 In cases that are vision-threatening, however, early intervention is recommended.
PVR-related RD repair is a complex vitreoretinal procedure with multiple factors at play, and careful planning is key to successful retinal reattachment and prevention of further proliferation. Lens status is an important factor to consider prior to surgery. In patients with visually significant cataracts or cataracts that will likely mature during the postoperative period and affect visualization of the posterior segment, consider cataract surgery with IOL placement. In patients with anterior PVR, surgeons should also consider lensectomy with capsule removal to allow for complete removal of anterior pathology. Another factor to consider preoperatively is placement of a scleral buckle. If a buckle wasn’t placed at the time of the previous surgery, consider placing one at the time of reoperation if you don’t plan on doing an extensive relaxing retinectomy.
In patients with complex RDs, it’s important to have a thorough discussion with the patient and key family members regarding the surgery and prognosis. Afterwards, give them plenty of time to ask questions, and address these questions. In patients in whom we’re planning to use silicone oil, we emphasize the multi-step nature of the process.
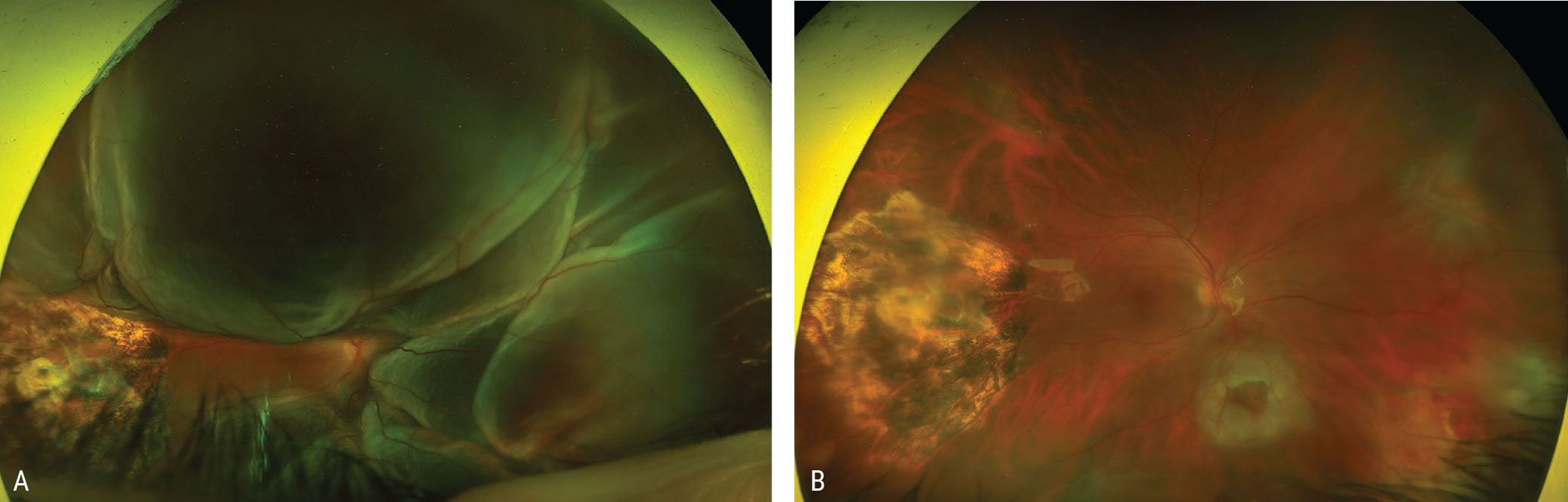
Intraocular preservative-free triamcinolone acetonide, such as Triesence (Alcon) can be used to highlight the vitreous, ensuring adequate removal of the posterior hyaloid and vitreous base. If cortical vitreous is present, it can be removed with a diamond-dusted membrane scraper (Synergetics) or a flexible nitinol loop (Alcon). Next, we meticulously remove all preretinal membranes. We may also peel the ILM, which can serve as a scaffold for PVR proliferation and potentially decreases recurrence of posterior ERM formation and recurrent detachment.18-20 We use indocyanine green or brilliant blue dye (TissueBlue, Dutch Ophthalmic USA) to stain the ILM, and peel posteriorly and as far out to the periphery as possible (Figure 1).
Subretinal PVR membranes—if isolated, extrafoveal and not exerting tractional forces—may not need to be removed for retinal reattachment. However, in cases requiring removal, we like to use chandelier illumination, placement of an extramacular retinotomy, and forceps in a hand-to-hand technique to remove the membrane.
After peeling, if the retina still appears stiff and unable to flatten, a relaxing retinectomy—a circumferential excision of retinal tissue—may be necessary to relieve traction. We try to perform the retinectomy as far anterior as possible, to preserve functional retina. It’s also important to incorporate any potentially problematic retina into the retinectomy, if not too posterior. We like the edge of the retinectomy to be healthy and free of PVR membranes, contracture and traction. We use diathermy to create a continuous line to delineate where we want to create the incision. Diathermy is also helpful for hemostasis. Careful steps should be taken to minimize bleeding during the retinectomy, as hemorrhage will carry blood-derived cytokines which can promote recurrent PVR. In very severe cases, a 360-degree retinectomy may be necessary to achieve retinal flattening.
Following a retinectomy, we use perfluorocarbon liquid (PFCL) to flatten the retina. When instilling the PFCL, tilt the eye away from the retinectomy to ensure the subretinal fluid is maximally squeezed out, and also to help prevent folding of the retinectomy edge. Then do a careful air-fluid exchange, starting anteriorly in the fluid phase and then draining the subretinal fluid starting at the anterior edge of the retinectomy and moving more posteriorly as the exchange progresses. Also, rotate the eye in the direction of the retinectomy to remove as much subretinal fluid as possible. At the end, place a few drops of balanced saline solution posteriorly to aspirate any residual PFCL.
 |
|
Figure 1. A) Preoperative fundus image of a 44-year-old man referred for proliferative vitreoretinopathy following vitrectomy for a posterior intraocular foreign body with an impact site temporal to the macula. The patient has a near total retinal detachment with multiple posterior and peripheral starfolds. B) Postoperative fundus image showing a successfully attached retina following vitrectomy and membrane peeling, retinotomy, drainage of subretinal fluid, endolaser and silicone oil. The preretinal membranes were peeled and the ILM was peeled over the macula to the periphery using ICG for visualization of the ILM. |
Excessive laser is unnecessary and can be proinflammatory. We typically place two rows of laser at the posterior edges of the retinectomy and reinforce the anterior points of the retinectomy at 3- and 9-o’clock with some additional laser using an illuminated endolaser and scleral depression.
In eyes with extensive PVR or large retinectomies, silicone oil tamponade is recommended. In eyes that are aphakic or where pupillary block is a concern, it’s important to place an inferior iridotomy when using silicone oil. Having the patient position face down postoperatively can be helpful in preventing development of folds.
Close follow-up and careful postoperative management following PVR surgery are essential for success and good visual outcomes. It’s important to watch for and treat intraocular inflammation, cystoid macular edema and hypotony. Patients may also develop epiretinal membranes or tractional macular edema which, if visually significant, can be treated to help enhance visual outcomes. Silicone oil-related issues include emulsification leading to ocular hypertension or band keratopathy. Early recognition and treatment of these complications is vital to maintaining the success of the PVR surgery and improving visual outcomes.
Future Directions
Several pharmaceutical agents have been employed over the years in attempts to reduce the rate of postoperative PVR recurrence, with variable success. Considering inflammatory mechanisms of PVR formation, corticosteroids present as a logical treatment of choice. However, studies have failed to show a difference in final visual acuity between treatment (systemic or intravitreal steroids) and control groups.21-23 Anti-neoplastic drugs, such as 5-fluorouracil and daunorubicin, displayed mixed results. In addition, their use is limited by systemic toxicity.
A promising agent in the treatment of severe postoperative PVR is the antimetabolite agent methotrexate. Weekly intravitreal methotrexate injections of 400 mcg/0.05 mL beginning intraoperatively have been successfully employed to reduce PVR- associated recurrent detachment in high-risk patients.24,25 Currently, a prospective, randomized clinical trial is under way that aims to assess the efficacy of postoperative intravitreal methotrexate in prevention of PVR-associated redetachment.26
In conclusion, modern advances in vitreoretinal surgery allow for successful retinal reattachment in most cases of PVR. However, despite anatomic success, visual outcomes are variable. Timely diagnosis, a thoughtful surgical approach and careful postoperative management are key to successful retinal reattachment and vision preservation.
Dr. Fromal is an ophthalmology resident at Wills Eye Hospital Residency Program at Thomas Jefferson University Hospital in Philadelphia.
Dr. Mehta practices at Mid-Atlantic Retina, the Retina Service of Wills Eye Hospital; and is an assistant professor at Thomas Jefferson University Hospital.
1. Charteris DG, Sethi CS, Lewis GP, et al. Proliferative vitreoretinopathy—developments in adjunctive treatment and retinal pathology. Eye 2002;16:4:369-374.
2. Kerkhoff FT, Lamberts QJ, van den Biesen PR, Rothova A. Rhegmatogenous retinal detachment and uveitis. Ophthalmology 2003;110:2:427-431.
3. Wiedemann P, Yandiev Y, Hui Y. Pathogenesis of Proliferative Vitreoretinopathy. In: Schachat AP, ed. Ryan’s Retina, 6th edition. Elsevier Health Sciences, 2017:5,658-5,690.
4. Chen Z, Shao Y, Li X. The roles of signaling pathways in epithelial-to-mesenchymal transition of PVR. Molecular Vision 2015;21:706.
5. Hiscott PS, Grierson I, McLeod D. Natural history of fibrocellular epiretinal membranes: A quantitative, autoradiographic, and immunohistochemical study. British Journal of Ophthalmology 1985;69:11:810-823.
6. Feng K, Wang CG, Hu YT, et al. Clinical features and prognosis of eyeball rupture: Eye injury vitrectomy study. Clinical & experimental ophthalmology 2015;43:7:629-636.
7. Nagasaki H, Shinagawa K. Risk factors for proliferative vitreoretinopathy. Current opinion in ophthalmology 1995;6:3:70-75.
8. Nagasaki H, Shinagawa K, Mochizuki M. Risk factors for proliferative vitreoretinopathy. Progress in retinal and eye research 1998;17:1:77-98.
9. Girard P, Mimoun G, Karpouzas I, et al. Clinical risk factors for proliferative vitreoretinopathy after retinal detachment surgery. Retina 1994;14:5:417.
10. Wickham L, Ho-Yen GO, Bunce C, et al. Surgical failure following primary retinal detachment surgery by vitrectomy: Risk factors and functional outcomes. British Journal of Ophthalmology 2011;95:9:1234-1238.
11. Scott IU, Flynn HW, Azen SP, et al. Silicone oil in the repair of pediatric complex retinal detachments: A prospective, observational, multicenter study. Ophthalmology 1999;106:7:1399-1408.
12. Hilton G, Machemer R, Michels R, Okun E, Schepens C, Schwartz A. The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology 1983;90:2:121-125.
13. Machemer R, Aaberg T, Freeman HM, et al. An updated classification of retinal detachment with proliferative vitreoretinopathy. American Journal of Ophthalmology 1991;112:2:159-165.
14. Di Lauro S, Kadhim MR, Charteris DG, et al. Classifications for proliferative vitreoretinopathy (PVR): An analysis of their use in publications over the last 15 years. Journal of Ophthalmology 2016;7807596.
15. Idrees S, Sridhar J, Kuriyan AE. Proliferative vitreoretinopathy: A review. International Ophthalmology Clinics 2019;59:1:221.
16. Pastor JC, de la Rúa ER, Martı́n F. Proliferative vitreoretinopathy: Risk factors and pathobiology. Progress in retinal and eye research 2002;21:1:127-144.
17. Coffee RE, Jiang L, Rahman SA. Proliferative vitreoretinopathy: Advances in surgical management. International Ophthalmology Clinics 2014;54:2:91-109.
18. Rao RC, Blinder KJ, Smith BT, Shah GK. Internal limiting membrane peeling for primary rhegmatogenous retinal detachment repair. Ophthalmology 2013;120:5:1102-1103.
19. Akiyama K, Fujinami K, Watanabe K, et al. Internal limiting membrane peeling to prevent post-vitrectomy epiretinal membrane development in retinal detachment. American Journal of Ophthalmology 2016;171:1-10.
20. Foveau P, Leroy B, Berrod JP, et al. Internal limiting membrane peeling in macula-off retinal detachment complicated by grade B proliferative vitreoretinopathy. American Journal of Ophthalmology 2018;191:1-6.
21. Koerner F, Koerner-Stiefbold U, Garweg JG. Systemic corticosteroids reduce the risk of cellophane membranes after retinal detachment surgery: A prospective randomized placebo-controlled double-blind clinical trial. Graefe's Archive for Clinical and Experimental Ophthalmology 2012;250:7:981-987.
22. Ahmadieh H, Feghhi M, Tabatabaei H, et al. Triamcinolone acetonide in silicone-filled eyes as adjunctive treatment for proliferative vitreoretinopathy: A randomized clinical trial. Ophthalmology 2008;115:11:1938-1943.
23. Banerjee PJ, Quartilho A, Bunce C, et al. Slow-release dexamethasone in proliferative vitreoretinopathy: A prospective, randomized controlled clinical trial. Ophthalmology 2017;124:6:757-767.
24. Eliott D, Stryjewski TP. Methotrexate for proliferative vitreoretinopathy. US Patent 2017.
25. Eliott D. Methotrexate for the prevention of PVR: A Phase 1 study. Presented at Vail Vitrectomy, Vail, Colorado, Feb 20, 2016.
26. The GUARD Trial – Part 1: A Phase 3 clinical trial for prevention of proliferative vitreretinopathy. Available at: https://clinicaltrials.gov/ct2/show/NCT04136366 (Accessed June 6, 2021)



