The chances of encountering a refractive surprise beyond 1.00 to 2.00 D following cataract surgery are pretty slim. Technological advances in keratometry and A-scan biometry instrumentation that help predict intraocular lens power calculations have eliminated the potential for major mistakes.
Yet, occasionally the bizarre happens. Your 66-year-old patient, who had 4 D of myopia before cataract surgery, now has 2 D of hyperopia postoperatively—a big shift in refraction that challenges you to find the best method to achieve your original goal: emmetropia.
 |
| A technician obtains a patient's axial-length measurement by A-scan immersion ultrasound to predict a more accurate intraocular lens power calculation. |
In the following article, ophthalmologists discuss the common causes of refractive surprise after cataract surgery, methods of prevention, and the surgical procedures they use to correct the errors.
Anatomy and Instrumentation
Ophthalmologists say there is one reason why refractive surprises continue to occur after cataract surgery procedures. Physicians simply don't have the appropriate instruments and formulas on hand to accurately measure a small percentage of eyes that are what they call anatomically "abnormal" or "unusual."
"Keratometers and ultrasonic devices have [built in] assumptions about the anatomy of normal eyes that they use to calculate measurements," says Jack T. Holladay, MD, MSEE, FACS, clinical professor of ophthalmology at Baylor College of Medicine, and president of Holladay LASIK Institute in Houston. "But the more unusual the anatomy of the patient's eye, the less likely those assumptions hold true. You're more likely to get a measurement error in an eye that's anatomically abnormal than in one that's normal," he says.
Warren Hill, MD, medical director of East Valley Ophthalmology in Mesa, Ariz., agrees: "Most people use two variable formulas based on axial length and keratometry, which perform best when the patient's measurements are close to those of a schematic eye. The assumption these formulas make is that the anterior and posterior segments are always proportional. And this is not necessarily so. You're sometimes going to get funny results."
Anatomically unusual eyes fall into the following three categories that are the most common causes of refractive surprise after cataract surgery.
| Hoffer Programs | Postop Rx Range |
| AL 21.52 Short: use HoffQ K1 44.50 K2 43.75 44.2, OK TrgRx 0 IOL Alcon IOL SAVE CLR SRK/T A Con 118.00 25.73 Holladay 1 SF 1.22 26.10 HofferQ ACD 4.97 26.34 26.0 (avg = 26.06) CALCULATE See Range |
IOL SRK/T Holl HoffQ 28.0 -1.73 -1.42 -1.23 27.5 -1.34 -1.04 -0.85 27.0 -0.96 -0.67 -0.48 26.5 -0.58 -0.29 -0.11 26.0 -0.20 0.08 0.25 25.5 0.17 0.44 0.61 25.0 0.54 0.80 0.97 OK |
| Left: The main calculation screen using the HofferQ, Holladay 1 and SRK/T formulas. Right: This screen shows refractive results of different IOL powers. | |
• Previous corneal refractive surgery. Prior corneal surgery such as radial keratotomy, automated lamellar keratoplasty, photorefractive keratectomy, LASEK or LASIK is "our number one cause of refractive surprise following a cataract procedure," says Karl Stonecipher, MD, director of Southeastern Laser and Refractive Center in Greensboro, N.C.
Most measurement devices are designed to measure corneas that are steeper in the center and flatter along the periphery, says David R. Hardten, MD, director of refractive surgery at Minnesota Eye Consultants, and adjunct professor of ophthalmology at the University of Minnesota in Minneapolis. The alteration in corneal curvature induced by refractive surgery is what throws off standard keratometry readings, giving you inaccurate corneal-power measurements. "And the keratometry reading is one of the most important factors in IOL-power calculations, second to the axial-length measurement," says Kenneth J. Hoffer, MD, FACS, clinical professor of ophthalmology at UCLA, Jules Stein Eye Institute in Los Angeles, and president of EyeLab Inc. and St. Mary's Eye Center in Santa Monica, Calif.
For instance, many people who've had RK become hyperopic after cataract surgery because of incorrect keratometry readings. "You aim for emmetropia, but your patient ends up a 1 D or 2 D hyperope," says Richard S. Hoffman, MD, clinical associate professor at Oregon Health & Science University, and an ophthalmologist in private practice in Eugene, Ore. "That's because they now have an oblate cornea instead of a prolate cornea," he says.
To get accurate readings, it's not just a matter of switching keratometers or topographers, either. Currently, there are no devices designed specifically to measure corneal power accurately after refractive surgery. "The [standard] instruments assume that the back surface of the cornea is 84 percent of the front surface," says Dr. Holladay. "In a normal eye, that's true. But once somebody's touched your cornea with LASIK or PRK, then that relationship is no longer true."
Two ways to get around this is to perform what Dr. Hoffer terms the clinical history method and the contact-lens method. The history method involves obtaining the patient's spherical equivalent refractive errors before and after refractive surgery, determining the difference between the two and subtracting that amount from the corneal-power measurement prior to the refractive surgery, says Dr. Hoffer.
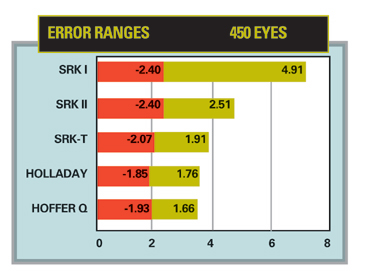 |
| Ranges of IOL power error in 450 eyes, using regression formulas compared to the HofferQ, Holladay and SRK/T formulas. |
The contact-lens method, which Dr. Hoffer believes may be more accurate than the history technique, entails refracting the patient's eye, determining the spherical equivalent [RB], then placing a hard PMMA plano contact lens of known base curve [B] on the eye that's closest to the corneal-power reading. Then, calculate the spherical equivalent of the patient's refraction with the contact lens on [RCL] and plug that information into a mathematical formula. "The estimated keratometry reading is equal to B+P+RCL-RB, where P is the power of the contact lens if not plano. So basically, it's the base curve of the contact lens plus the power of the contact lens plus the refraction with the contact lens on minus the bare refraction," says Dr. Hoffer.
Trouble is, most ophthalmologists don't take the time to perform the contact-lens method, he says, so they end up with more refractive surprises after cataract surgery than are necessary. "All they have to do is be prepared by having three contact lenses available in the office for this purpose ahead of time," he adds.
• Long eyes. Eyes with axial lengths of 26.5 mm or longer make it impossible for standard ultrasound devices to calculate accurate measurements, because they often have posterior staphylomas, says Dr. Holladay. "You want to measure the axial length to the fovea, but [the ultrasound device] measures the axial length to the posterior pole, or pit of the staphyloma instead, interpreting the axial length as longer than it really is," he says.
If the axial length measures longer than it actually is, you'll mistakenly use an implant with less power than you need, leaving the patient hyperopic after cataract surgery, says Dr. Hardten. And if the axial length measures shorter than what it really is, you may use an implant with a higher power than what you need, causing myopia after the cataract procedure.
Dr. Stonecipher recalls a case involving a 55-year-old patient who had -31 D of myopia and a posterior staphyloma prior to cataract surgery. "Following the cataract procedure, she was undercorrected by a mean spherical equivalent of roughly -4 D," he says. Two weeks later, he implanted a piggyback IOL, bringing her closer to emmetropia. "Although we're good with IOL-power calculations, at the extreme ends we still have issues," he says. "We don't have enough patients at these numbers or a good nomogram to adequately calculate IOL power, or their eyes are so short or so long that we run into difficulty measuring them, especially in the severely myopic individuals, in whom posterior staphylomas are much more common. They have such anatomically different eyes."
 |
| A B-scan (left) shows a staphylomatous eye. The A-scan (upper right) shows a shorter reading at the macula than at the posterior pole (lower right). |
The solution to accurately measuring staphylomatous eyes is to use B-biometry, says Dr. Hill. "A vector A- and B-scan ultrasound device will perform a B-scan and simultaneous A-scan to get an image of the back of the eye where the optic nerve is," he says. "With that image on screen, you electronically move the cursor to where the retina is and measure right to the fovea. That gives you the refractive axial length, which is what you want, rather than the anatomic axial length."
Even better is the IOLMaster, says Dr. Hill. "As long as the patient is able to look directly at the little fixation light, the measurement is directed to the center of the fovea, giving you the refractive axial length."
• Short eyes. Nanophthalmic eyes measuring under 22 mm pose different problems than longer eyes in that there's less room for making even the slightest of errors in measurements. For instance, if you make a 1-mm error in your axial-length measurement in a normal eye that's 23.5-mm long, you'll end up with a -2.50-D surprise, says Dr. Holladay. But if you make that same 1-mm error in an eye that's 16 or 17 mm in length, you'll have a
-4-D error. "So the shorter the eye, the more precise the measurement must be to maintain the same percentage of error as the long eye," he says.
Dr. Hoffer agrees: "Short eyes are always a problem. The mathematics of the optical formulas cause greater errors because everything is so small."
Axial lengths below 22 mm are often seen in hyperopes who, like others with short eyes without hyperopia, have abnormal ratios of the anterior-chamber depth to the length of the eye. "Some hyperopic patients have normal eyes with regard to the position of the crystalline lens, but some have very shallow anterior-chamber depths," says Dr. Hoffman. "So if you use the older IOL formulas, many of your patients will still end up hyperopic post cataract surgery," he adds.
Another cause of refractive surprise after a cataract procedure is incorrect lens position. Effective lens position, or ELPo, is the final variable influencing emmetropia, myopia or hyperopia after cataract surgery, says Dr. Hardten. "If you predict that the implant is going to sit farther forward, based on your IOL-power calculation formulas, then you'd put in an implant of lesser power. However, your patient may end up more hyperopic after surgery if the implant sits farther backward than you had planned," he says. "If you predict that the implant is going to sit farther backward, you may put in an implant of greater power, but if it sits farther forward than you had expected, the patient will be more myopic after the surgery."
To better predict ELPo, physicians must measure the horizontal corneal diameter (white to white), the anterior-chamber depth and the lens thickness. "These three measurements give you the additional information you need to better predict where the lens will end up in the eye," says Dr. Holladay. "An anterior-chamber lens needs to be about 1.50 D to 2 D less than the equivalent posterior lens. So the power of the lens is not only dependent on the length of the eye but also on where it sits in the eye," he explains.
Other factors causing refractive surprise include mislabeling of the IOL- implant power by the manufacturer and errors made in the operating room, where someone accidentally hands the surgeon the wrong lens. "But these incidents are rare today," assures Dr. Holladay. "They occur so infrequently that they are insignificant."
Tackling the Surprises
Whether or not physicians figure out why an error occurs, there's always plan B: choosing the best surgical procedure to correct the problem.
Here are the most common methods of correction that ophthalmologists use.
• Corneal refractive surgery. If a patient has a low refractive error of 1.50 D to 2 D of hyperopia or up to 3 D of myopia, Dr. Hardten says he performs a LASIK procedure to correct the surprise. If there's any evidence of anterior basement membrane dystrophy or dry-eye syndrome, he says he leans more toward PRK. Additionally, he says he considers CK in the low hyperope.
Harry Grabow, MD, assistant professor of ophthalmology at the University of South Florida, and medical director of the Sarasota Cataract and Laser Institute and Center for Advanced Eye Surgery in Sarasota, Fla., says he prefers RK for the low myope with -2 D to -3 D of refractive error. "RK also works very well in older patients for higher degrees of myopia up to -5 D," he says. For astigmatism, he performs astigmatic keratotomies or limbal relaxing incisions. Dr. Hoffman also uses LRIs for astigmatism and combines them with toric IOLs when the refraction is greater than 3 D.
Dr. Stonecipher says he performs AK or RK on patients with postoperative astigmatism or myopia of 1.50 D or less, or LASIK if the refractive error is between -1.50 D and -3 D. If the error is greater than -3 D, he recommends an IOL exchange or a piggyback lens if there are no contraindications preventing an intraocular procedure. He usually suggests CK for patients with 1.25 D or less of hyperopia, and LASIK if they're between 1.25 D and 3 D. Dr. Hoffer says he doesn't recommend corneal refractive procedures to repair refractive surprises after cataract surgery. Instead, he says he prefers lens exchanges or piggyback IOLs. In the future, he says he'll choose ICLs or PRLs rather than an IOL implant.
• Piggyback IOLs. These lenses are preferred by physicians if a patient's refractive surprise exceeds 3 D, or if she's had previous corneal refractive surgery. Dr. Stonecipher uses piggyback IOLs "if there's a contraindication for operating on the patient's cornea for whatever reason. If somebody has had a maximum RK, has a thinner corneal thickness, or has had a big LRI, I'll use a piggyback IOL," he says. Dr. Grabow uses piggyback IOLs for correcting myopia and especially hyperopia. "They are much more accurate than IOL exchanges and are less traumatic," he says.
• IOL exchange. Replacing a lens with a supposedly more accurate one to correct a refractive surprise is a last resort for many ophthalmologists, says Dr. Grabow. "There's a higher degree of error when trying to achieve the refractive result when compared to other corrective modalities. And an IOL exchange has a higher potential for surgical complications than the other methods," he says.
There's more room for additional error when doing a lens exchange because "you're basing your error on the assumption that you know the lens in the eye is correct," says Dr. Holladay. "You may put in what you think is a
+20-D lens, and you come out with a -4 D surprise. You figure if the guy was -4 D with a +20-D lens, then a +16-D lens will be just right. Then you put in the +16-D lens, and now he's a +4 D hyperope, because the first lens you put in was mislabeled in the first place," he says.
"Or, the axial length might be off. The keratometry readings might be off. The lens might be deeper in the eye. All of these are possibilities for the refractive surprise. So if you do a lens exchange, and you don't know which of these is wrong, you'll make the same mistake again. So I recommend implanting a piggyback IOL, because you're putting in a lens to correct the residual surprise. It doesn't matter what the original lens power was or what caused the error, because you're fixing it," he says.