Knut Eichhorn-Mulligan, MD, PhD and Ivana K. Kim, MD, Boston
Choroidal nevi, especially small ones, are common. Estimates of their prevalence range from 5 to 10 percent of the general population. The Blue Mountains Eye Study recorded choroidal nevi in 6.5 percent of a prospectively studied population of mostly middle-aged white Australians.1 Choroidal melanomas, on the other hand, are fortunately a rare entity, with an estimated incidence of approximately six per million. Nevertheless, choroidal melanoma is the most common primary intraocular malignancy in adults, and prompt and accurate diagnosis is important.
Characteristics of Nevi
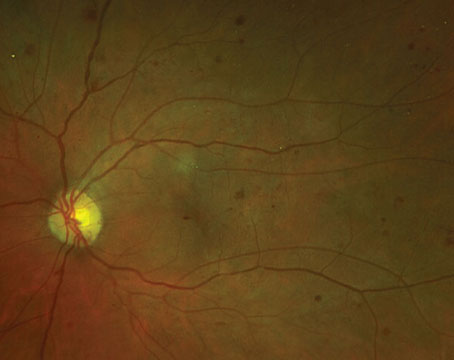
Histologically, nevi are benign neoplasms of the melanocytes that reside in the outer layers of the choroid. They typically appear as a flat, slate-grey lesion beneath the retina. However, nevi may be completely nonpigmented in up to 10 percent of cases.2 The risk of malignant transformation is estimated to be quite low.3 Nevertheless, nevi have the potential to cause visual dysfunction, especially if they are located near the fovea. By compressing the overlying choriocapillaris, thicker nevi may obstruct blood flow to the RPE and outer retinal layers and lead to RPE and photoreceptor degeneration.4 Nevi have been associated with visual field defects in a surprisingly large number of patients, although if peripheral, these deficits may not become symptomatic.5,6 More visually significant complications that have been observed in association with benign nevi include serous detachments of the RPE and retina and choroidal neovascularization.4 A recent review of visual function in more than 3,400 patients with nevi at 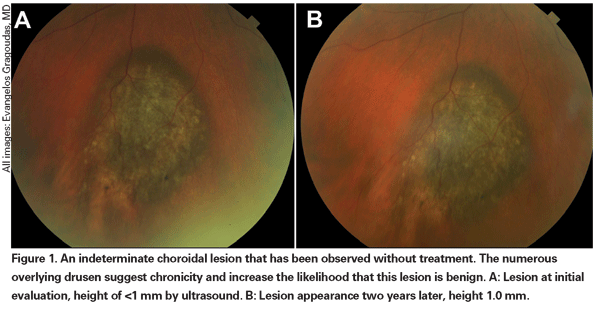
Whether melanomas arise from pre-existing nevi, or are in fact a separate entity, is somewhat controversial. Analysis of enucleated eyes has demonstrated histological features characteristic of nevi within the edges of malignant melanomas, suggesting that at least some melanomas may evolve from malignant transformation within pre-existing nevi.8 Nevertheless, the risk that a choroidal nevus may turn into a melanoma is generally considered extremely low. Estimates of annual rates of malignant transformation vary from between one in 4,800 to one in 8,800.3,9
In general, small choroidal nevi rarely enlarge. In the Blue Mountains study, out of 160 nevi (mean diameter 1.25 mm), for which at least five years of follow-up was available, only one showed a slight increase in diameter.10 However, there are several reports of histologically confirmed choroidal nevi that had shown progressive enlargement, but lacked any malignant features on careful review.11,12 Furthermore, in an analysis of more than 2,500 eyes followed in a tertiary referral practice, Carol Shields, MD, and colleagues noted enlargement in 3 to 4 percent of nevi without characteristics of malignant transformation.2 The nevi in this clinic-based cohort were significantly larger at baseline than those in the population-based Blue Mountains study, averaging approximately 5 mm in diameter.

Detecting Small Melanomas
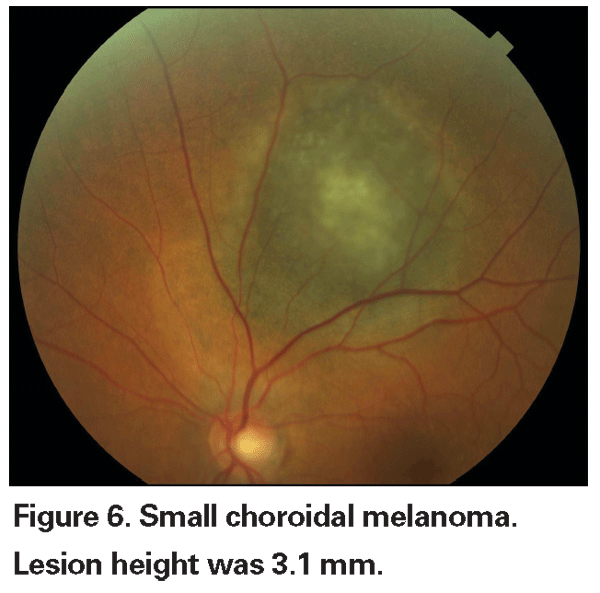
Pigmented choroidal lesions that are mildly elevated may be called indeterminate lesions and present a challenge with regard to diagnosis and management. Given the risks and limitations with respect to obtaining histological confirmation of malignancy, ophthalmologists need to rely on clinical characteristics identified as predictive of growth and metastasis in order to differentiate small melanomas from elevated choroidal melanocytic tumors that are likely benign. Dr. Shields and colleagues identified five factors associated with risk of growth of small choroidal melanocytic lesions less than 3 mm in thickness from retrospective analyses of approximately 1,300 patients.13 These factors were: 1) tumor thickness greater than 2.0 mm; 2) subretinal fluid; 3) visual symptoms; 4) orange pigment; 5) posterior tumor margin touching the disc. Lesion growth was observed in 4 percent of patients with none of these risk factors, 36 percent of those with one risk factor, and in more than 50 percent of those with three or more factors.14 Clinical factors associated with an increased risk of metastasis included: 1) posterior margin touching the disc; 2) documented growth; and 3) increased tumor thickness (greater than/equal to 1.1 mm).13 The small-tumor observational study conducted by the Collaborative Ocular Melanoma Study (COMS) Group identified similar risk factors associated with tumor growth; namely 1) greater apical tumor thickness, 2) larger initial basal diameter, 3) presence of orange pigment, 4) absence of drusen, and 5) absence of retinal pigment epithelial change adjacent to the tumor.15 These last two factors confirm the clinical observation that the presence of drusen and pigment alterations are indicators of chronicity, and therefore are more likely to be seen over dormant, benign lesions. 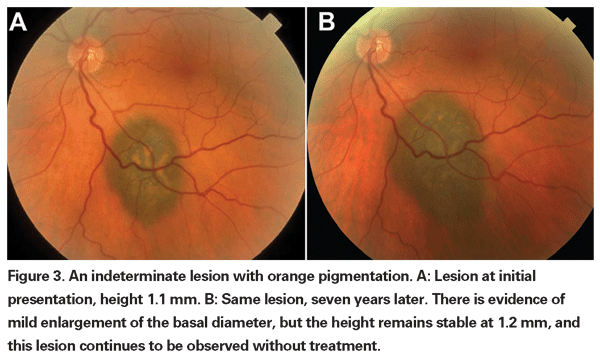
Imaging characteristics may also be helpful in evaluating the potential for malignancy. The presence of an internal quiet zone on B-scan ultrasonography and hot spots on fluorescein angiography have been shown to be predictors of tumor growth.16 The role of optical coherence tomography in evaluating choroidal lesions is also being explored. Gabriella Espinoza, MD, and colleagues have proposed that OCT may be valuable in differentiating between active subretinal fluid, where the retina overlying the lesion is elevated but otherwise normal in appearance, and chronic changes such as retinal thinning or intraretinal cysts. In their small series, they found a correlation between an active subretinal fluid on OCT and documented tumor growth.17
Early Treatment vs. Observation
When enucleation was the only accepted form of treatment for melanoma, observation until documented growth was advocated for small tumors that could not be definitely diagnosed as melanomas on initial presentation. Now with the availability and demonstrated efficacy of globe-sparing therapies, an argument can be made for earlier treatment of these indeterminate lesions. Data from the COMS trials reveals that melanoma-related mortality varies with tumor size at time of treatment. For medium tumors (defined as tumors 2.5 to 10 mm in apical height and up to 16 mm in largest basal diameter), melanoma-specific mortality was 10 percent at five years, and 18 percent at 10 years.18 For large tumors (those exceeding the size criteria for medium tumors in either apical height or largest basal diameter; or peripapillary tumors with an apical height greater than 8 mm), the rates increased to approximately 27 percent at five years and 40 percent at 10 years.19 Additionally, as mentioned above, documented growth prior to treatment has been shown to be a risk factor for metastasis. However, growth may be a marker for more aggressive tumors, and it has not been proven that treating these tumors earlier reduces mortality.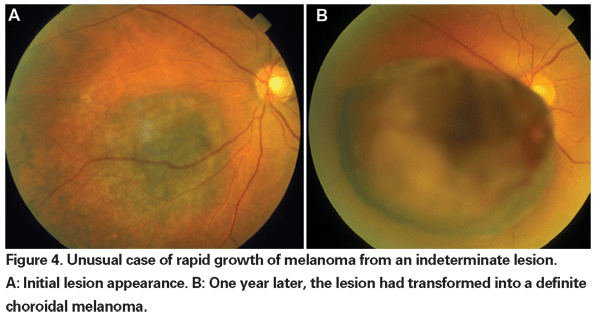
Our current methods of radiotherapy allow for effective local tumor control with globe conservation, but visual morbidity is still high. Therefore, it is necessary to weigh the mortality risk incurred by careful observation prior to treatment of indeterminate lesions against the consequences of visual loss induced by therapy. In the COMS small-tumor observational study, there were six melanoma-related deaths out of a group of 67 patients with tumors that were treated after an initial period of observation.20 Only two of these deaths occurred within five years after enrollment, resulting in an approximate five-year melanoma-specific mortality rate of 3 percent. More recently, investigators at the Bascom Palmer Eye Institute reported a five-year melanoma-specific mortality rate of 3.9 percent in a small group of patients with suspected choroidal melanoma observed for growth or development of orange pigment prior to treatment with plaque radiotherapy.21 These studies suggest low mortality rates associated with delayed treatment for indeterminate lesions. Additionally, a small retrospective series showed no increase in melanoma-specific mortality between promptly treated and initially observed patients.22 To date, there is no evidence to demonstrate a survival benefit from earlier versus delayed treatment of small tumors that cannot be definitively diagnosed as melanomas. Therefore, it is not clear that sacrificing vision in a greater number of patients with indeterminate lesions will definitely save more lives.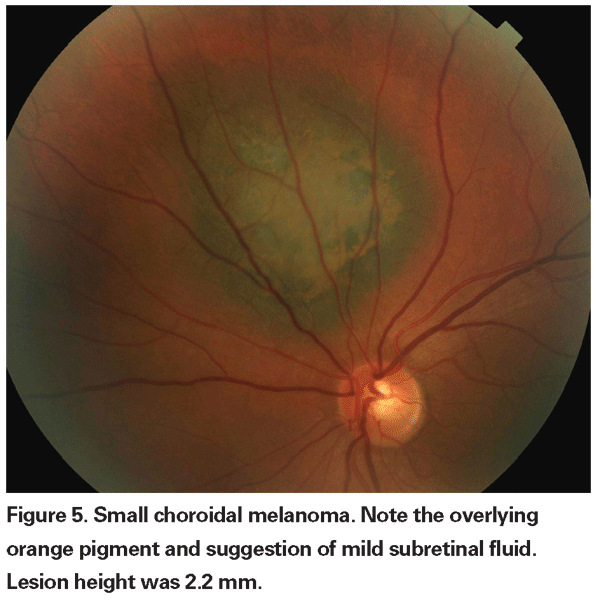
To summarize, choroidal pigmented lesions may be grouped into three categories: 1) small, flat lesions that are clearly choroidal nevi; 2) small lesions with elevation <2.5 mm that may have orange pigment or subretinal fluid or one of the other factors that raise suspicion for malignancy--the so-called indeterminate lesions; and 3) dome- or mushroom-shaped choroidal tumors greater than/equal to 2.5 mm that are clearly melanomas. It is the treatment of the indeterminate lesions that still remains an area of controversy, and evaluation and management of these lesions is best performed by ocular oncologists. A clinical trial comparing early vs. delayed treatment for indeterminate lesions may not be feasible due to the large number of patients and length of follow-up necessary for conclusive results. With ongoing advances in molecular diagnostic techniques, the best hope for increasing survival of patients with choroidal melanoma lies in the development of effective treatments for metastatic disease and in the search for biomarkers that might more precisely predict malignant transformation.
Dr. Eichhorn-Mulligan is in residency in ophthalmology at
1. Sumich P, Mitchell P, Wang J. Choroidal nevi in a white population - the Blue Mountains Eye Study. Arch of Ophthalmol 1998;116: 645-650.
2. Shields CL, Furuta M, Mashayekhi A, Berman EL, Zahler JD, Hoberman DM, Dinh DH, Shields JA. Clinical spectrum of choroidal nevi based on age at presentation in 3,422 consecutive eyes. Ophthalmology 2007: Dec 5 [Epub ahead of print].
3. Singh AD, Kalyani P, Topham A. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology 2005;112: 1784-1789.
4. Gonder JR, Augsburger JJ, McCarthy EF, Shields JA. Visual loss associated with choroidal nevi. Ophthalmology 1982;89:961-965.
5. Tamler E, Maumenee AE. Clinical study of choroidal nevi. Arch Ophthalmol 1959;62:196-202.
6. Flindall RJ, Drance SM. Visual field studies of benign choroidal melanomata. Arch Ophthalmol 1969;81:41-44.
7. Shields CL, Furuta M, Mashayekhi A, Berman EL, Zahler JD, Hoberman DM, Dinh DH, Shields JA. Visual acuity in 3,422 consecutive eyes with choroidal nevus. Arch Ophthalmol 2007;125:1501-1507.
8. Yanoff M, Zimmerman LE. Histogenesis of malignant melanomas of the uvea. II. Relationship of uveal nevi to malignant melanomas. Cancer 1967;20:493-507.
9. Ganley JP, Comstock GW. Benign nevi and malignant melanomas of the choroid. Am J Ophthalmol 1973;76:19-25.
10. Thiagalingam S, Wang JJ, Mitchell P. Absence of change in choroidal nevi across 5 years in an older population. Arch Ophthalmol 2004;122:89-93.
11. MacIlwaine WA,
12. Elner VM,
13. Shields CL, Shields JA, Kiratli H, De Potter P, Cater JR. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology 1995;102:1351-1361.
14. Shields CL, Cater J, Shields JA, Singh AD, Santos MCM, Carvalho C. Combination of clinical factors predictive of growth of small choroidal melanocytic tumors. Arch Ophthalmol 2000;118:360-364.
15. The Collaborative Ocular Melanoma Study Group. Factors predictive of growth and treatment of small choroidal melanoma. Arch Ophthalmol 1997;115:1537-1544.
16. Butler P, Char DH, Zarbin M, Kroll S. Natural history of indeterminate pigmented choroidal tumors. Ophthalmology 1994;101: 710-716.
17. Espinoza G, Rosenblatt B, Harbour W. Optical coherence tomography in the evaluation of retinal changes associated with suspicious choroidal melanocytic tumors. Am J Ophthalmol 2004;137:90-95.
18. The Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol 2006;1684-1693.
19. Hawkins BS, The Collaborative Ocular Melanoma Study Group. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma: IV. Ten-year mortality findings and prognostic factors. COMS report no. 24. Am J Ophthalmol 2004;138:936-951.
20. The Collaborative Ocular Melanoma Study Group. Mortality in patients with small choroidal melanoma. Arch of Ophthalmol 1997;115:886-893.
21. Sobrin L, Schiffman JC, Markoe AM,
22. Augsburger JJ, Vrabec TR. Impact of delayed treatment in growing posterior uveal melanomas. Arch Ophthalmol 1993;111:1382-6.