|
Proactive Preparation
Clearly, the best way to manage intraoperative complications is to do two things: First, take steps ahead of time to minimize the risk of an occurrence; and second, be prepared should a setback occur. Along those lines, Audrey R. Talley Rostov, MD, cornea, cataract and refractive surgeon and partner at Northwest Eye Surgeons in Seattle, offers these suggestions.
• As much as possible, anticipate problems. “There are several ways to prepare for potential problems beforehand,” notes Dr. Rostov. “First, if you know this will be a more complicated case than normal, one that you don’t encounter very often, such as a sutured IOL, review the surgery with your staff ahead of time.
“Second, know when to use special equipment to prevent a complication from arising,” she continues. “For example, in a very young patient the capsule is much more elastic and the capsulorhexis will be much more difficult. If you have a femtosecond laser available to do the capsulorhexis, that might be a good situation in which to use it.
“Third, have special tools available,” she says. “If the patient is in his 90s or is very young, has a rock-hard nucleus or a traumatic cataract, or has a suspected or visible zonular dehiscence or dialysis, you want to have all the tools you might need to deal with those situations prepared ahead of time. For example, if a patient has a very advanced, dense, white or brunescent cataract, then I want to have Malyugin rings, iris hooks, Trypan blue, intraoperative epinephrine and a vitrectomy setup available in the room to help in the event of a complication. Obviously you don’t need those for every single case, but if you have the equipment easily available your OR staff doesn’t have to go hunting for it.
“Fourth, always have a backup lens available in case there’s a problem with the bag or the zonules,” she says. “This isn’t a common situation, but you need to be prepared for it. Probably the best sulcus lens is the STAAR AQ2010, because the length is 13.5 mm rather than 13 mm. At least have a three-piece IOL available as a backup; you can use those in the sulcus, as long as you’re mindful of their limitations. Hopefully by now every surgeon knows never to place a one-piece acrylic IOL in the sulcus.”
• Use alternate techniques in difficult circumstances. “Complications can arise if you stick to standard protocol when the situation is nonstandard,” notes Dr. Rostov. “For example, if a nucleus is very dense and adherent to the capsule, using phaco chop can lead to complications. In that situation, I sculpt as much as I can and make an enormous bowl that will then collapse upon itself. I can then use viscoelastic to viscodissect it from the posterior capsule. This significantly reduces the likelihood of a complication.”
• Avoid having your view obscured. “Sometimes when the assistant is squirting BSS on the cornea, it can obscure your view momentarily,” notes Dr. Rostov. “During that momentary obscuration of your view, you could end up phacoing the capsule or putting a hole in the posterior capsule or grabbing onto the anterior capsule. The solution is to make sure your assistant just irrigates very briefly, and only when you ask for it. Then, you’ll know when to anticipate it and your view won’t be obscured when you’re not expecting it, such as in the middle of an important maneuver.”
• Be alert for warning signs. “Whenever something occurs that seems out of the ordinary, stop and take a moment,” she says. “Look around and be very aware of what’s going on. That’s especially important in cases that are not routine or have the potential to be more complicated.”
Corneal Complications
|
• Corneal abrasions. “Corneal abrasions can happen during wound creation or as the result of an instrument slipping across the eye, for example when inserting the speculum,” says Robert Weinstock, MD, director of cataract and refractive surgery at the Eye Institute of West Florida in Largo, Fla. “Just about any instrument in cataract surgery is capable of causing an epithelial abrasion, and sometimes an epithelial abrasion can actually obstruct the surgeon’s view during the surgery.
“Depending on the size and location of the abrasion, the surgeon has several options for handling this,” he continues. “One option is to place a cohesive viscoelastic on the cornea to improve the surgical view and mask the abrasion. Another option is to debride the central epithelium, but this is usually done as a last resort, and only if there is a severely limited view into the eye because of a hazy or damaged epithelium.
“At the end of the case I recommend a soft contact lens be placed on the eye to avoid severe pain and aid in healing,” he adds.
• Wound burn. “Wound burn is not as common these days with advanced phaco power modulation and laser cataract softening, but it’s still possible if you have a very dense nuclear piece that gets stuck in the phaco needle handpiece or tubing and blocks aspiration flow out of the eye,” says Dr. Weinstock. “If you’re in foot position three and there’s no fluid moving out of the eye through the needle, it will heat up enough to cause thermal damage to the cornea. If this happens, it can be pretty devastating.
“In order for this chain of events to take place, something must be clogging the phaco needle, handpiece or tubing,” he continues. “Sometimes, a clog is caused by a thick dispersive viscoelastic or a particle of the nucleus. If you step on the phaco pedal when a clog prevents movement of fluid to cool the phaco needle you may end up with a wound burn, even if you’re using one of the new phaco machines that has pulse modalities. There are usually some tip-offs that a problem is occurring; for example, you may see plumes of white smoke in the anterior chamber, and nothing seems to be evacuating out of the eye through the phaco needle. You’ll probably also note that the cornea starts to get a whitish, coagulated look to it, usually on the anterior lip of the wound. It’s usually ‘game over’ once you see this.
“If you suspect a clog or occlusion you have to stop immediately,” he says. “Take the phaco needle out of the eye and flush the tip, handpiece and tubing. Most of time I find the culprit is a piece of dense nucleus stuck in the tubing. ”
| ||||||
“A wound burn will also induce a tremendous amount of astigmatism,” he adds. “What you usually have to do is help the patient tolerate the astigmatism for a few months, because in general the astigmatism will melt away as the cornea heals and remodels itself. Immediately post-surgery, the astigmatism may be 3, 4 or 5 D, but it will usually come down to 1 or 2 D within two or three months—although in some cases it may take as long as six months. Because wound burns take so long to heal, they are one of the biggest headaches a surgeon can encounter.”
“The best way to manage a wound burn is to avoid creating one,” notes Amar Agarwal, MD, director of Dr. Agarwal’s Eye Hospital in Chennai, India, who has pioneered numerous surgical procedures, including bimanual phaco. “One thing that will help prevent this problem is to have your assistant keep applying fluid to the cornea. If your assistant does this while you’re operating, you will never get a corneal burn. However, always be alert so that if you do get a wound burn you’ll diagnose the problem immediately.
“If a burn occurs, have the assistant keep putting fluid on the eye while you finish the surgery so that further burn does not occur,” he adds. “After the surgery, put a suture on that area to prevent the wound from gaping. If necessary, suture the incision, make another incision in another area and complete the surgery there.”
• Stripping and scrolling of Descemet’s membrane. “Although rare, this can happen during cataract surgery, depending on the instrumentation you’re using and how dense the cataract is,” says Dr. Weinstock. “You can usually notice it while you’re operating.
“It generally happens in one of two ways,” he explains. “The first is a scroll of tissue at the wound, from your incision or as a result of manipulating things through the wound. I see it a little more often in bimanual surgery because the instruments are a little bit rougher on the tissue; there are some sharper edges, especially with the irrigating choppers and the bare phaco needle. If you do see a little bit of a scroll, you can usually address it at the end of the surgery case by going though the secondary wound and gently irrigating the scroll of tissue back into position. It will usually stick there. Alternatively, you can use viscoelastic to manipulate it into position, or even a Kuglen hook.
“Sometimes a little piece of tissue comes off altogether, right underneath the wound,” he says. “If that happens, you’ll see a scalloped edge where it’s missing. In my experience, other cells migrate over to that area within a couple weeks and the cornea goes back to its normal thickness without any long-term complications.
“However, this situation raises issues concerning hydration,” he continues. “If you’re hydrating the cornea to thicken it and seal the wound, it’s already going to be a little swollen in that area because of the loss of endothelium; as a result, it could take longer than normal to dehydrate afterwards. That means you need to be gentle with your hydration if you see a piece of Descemet’s missing. In fact, if you see damage to the endothelium under the wound, you might consider placing a suture instead of overhydrating the cornea to get the wound to seal. The good news is that peripheral corneal swelling doesn’t usually impact vision at all.”
|
“If you see that a cut or slice has created a flap of Descemet’s and you have more surgery left to do, stop, come out of the eye and put in a dispersive viscoelastic; use it to push that flapping piece of endothelium and Descemet’s back into position. Hopefully it will stay in place for the rest of the case,” he says. “Sometimes you have to play around with it a little bit. It’s almost like a jigsaw puzzle; you have to manipulate the little flap of tissue so that it fits back into the space that was left when it tore out. You should also leave a thin layer of viscoelastic at the end of surgery so the tissue stays in place.
“Of course, using air to manipulate the torn tissue would not be a good choice during surgery because you wouldn’t be able to see well enough to complete the surgery,” he adds. “However, if you’re at the end of the case and you see some of Descemet’s membrane flapping centrally or partially detaching, you can use a large air bubble in the anterior chamber to push the layer of tissue back into its anatomical position, just as you would in a DSAEK case. Then you can have the patient lie in a supine position overnight and check him the next day.”
• Ballooning of the conjunctiva. “This usually indicates that you made your wound too far posteriorly and irrigation is flowing under the conjunctiva,” says Dr. Weinstock. “Wound leakage during irrigation causes the ballooning. If the ballooning gets severe, you can make an incision in the conjunctiva a few millimeters back from the cornea and release the fluid from the sub-conjunctiva and/or sub-Tenon’s space. If you think the problem is putting your surgery and visibility at risk, you can close that wound and make a new incision.”
Iris Complications
The iris can easily become involved in unfortunate developments during cataract surgery.
• Damaging the iris. “It’s possible to damage the iris with the phaco needle, especially in a small-pupil case,” notes Dr. Weinstock. “You can unintentionally grab the iris, leading to hemorrhages, bleeding and damage. Once the phaco needle engages the iris, the damaged tissue becomes even more floppy and can easily prolapse out of the wound, or continue to be sucked into the phaco tip or aspiration tip.
|
• Iris prolapse. If the wound is too big or the wound architecture is not just right, the iris can prolapse out onto the conjunctiva, causing damage to the iris and challenges for the surgeon.
“Floppy iris syndrome is probably the number one risk factor for iris prolapse, especially when coupled with poor wound construction,” says Dr. Weinstock. “If the wound is too short or not biplanar or triplanar, the iris can easily pop out. Another risk factor is having the wound size not match up well with the size of your instruments. If the wound is too big relative to the phaco needle and the sleeve you’re putting through it, you’ll leave extra room for fluid to squeeze out—and the iris will follow the fluid. That’s one reason I use bimanual surgery; the wound size matches the instrument exactly. In addition, the wounds are very small, so there’s physically no way for the iris to get out of the eye.
“If the iris does prolapse, act quickly,” he continues. “The longer the iris stays out of the eye, the more iris tissue you’ll lose and the less chance the iris will look natural afterwards. It will have transillumination defects and the sphincter muscle will be damaged, and the incident could have a very irregular, visually significant cosmetic impact.
“If the iris prolapses, one strategy that can buy you a little time is to put a dispersive viscoelastic such as Healon GV or Viscoat into the eye subincisionally and blow the iris back into the wound posteriorly,” he notes. “This can hold it in place temporarily by acting as a blockade. If you feel that there’s a risk of destroying the iris, another strategy is to use viscoelastic to push the iris back into the eye, suture the wound and make a fresh wound with better architecture next to it.”
Dr. Weinstock points out that one factor that can lead to an iris prolapse is having the pressure inside the eye too high at the end of the case. “Overinflation at the end of surgery—especially if the patient has floppy iris syndrome—can blow the iris anterior and out through the wound, or wounds,” he explains. “If you see this happening, before you try to use viscoelastic or BSS to push the iris back in, go to your smaller wound and decompress the eye to soften it. This will relieve the pressure and the iris usually falls back in.
“If I have wound prolapse during a case, I almost always put in a suture at the end of the case,” he adds. “That’s because if the eye were to collapse postoperatively, that iris is going to come right out. It has already shown a tendency to do that, and it’s already weakened.”
• The syringe tip comes off. “This is most likely to occur if you use a non-Luer-locked syringe, or fail to check that the connection is secure before surgery,” notes Dr. Weinstock. “If the syringe tip comes off in the eye it can cause damage and hemorrhaging inside the eye, possibly an iridodialysis where it rips the iris root away, or a cyclodialysis cleft. In theory the needle could even penetrate through the iris and tear the retina. If you ever encounter this during surgery, make sure to do a peripheral retinal evaluation afterwards and check everything inside the eye. Treat the accident as a penetrating trauma.
“Obviously, the best solution here is prevention,” he adds. “Use Luer-locked syringes and make sure that the tip is screwed down very tight. Double-check that yourself, every time you go into the eye. The most common time for this to happen is if there is a clog in the cannula tip, so always ensure that the cannula is patent with a quick test squirt before you enter the eye.”
Capsule-Related Concerns
Some of the most potentially devastating complications involve the anterior or posterior capsule or the zonules.
• If the capsulorhexis veers outward. “If this happens, the way you respond should depend partly on what you think is the cause,” says Dr. Rostov. “For example, it could mean that the chamber is shallowing. In that case, adding more viscoelastic can be helpful. It could indicate that something is raising the patient’s IOP. Is the patient’s blood pressure going up? Is the lid speculum causing undue pressure on the eye? Is the patient holding his breath? Is the patient anxious? If you suspect the latter, a little more IV sedation on board can be helpful.
|
• Zonular issues. “In most cases, you’ll know ahead of time that the zonules are going to be an issue,” says Dr. Rostov. “This is more likely in a patient with exfoliation, a smaller pupil, a traumatic cataract or a very dense cataract. If you do run into trouble, be generous with your viscoelastic and use capsular tension rings and/or segments as necessary. Don’t try to pull the bag away from the area of weakness; pull toward the area of weakness, so you don’t cause a bigger problem.”
• Managing a posterior capsule rupture. “If you notice a sudden deepening of the anterior chamber, that may indicate a posterior capsular tear or rupture,” Dr. Rostov points out. “If that occurs, be sure you don’t remove the irrigating handpiece from the eye. You want to keep the pressure up inside the eye; you don’t want to withdraw it and suddenly change the pressure gradient.”
Dr. Agarwal notes that if he’s doing phaco and the posterior capsule ruptures with the nucleus still inside the bag, he has several options. “One option is to extend the incision and remove the nucleus,” he says. “A second option is to extend the incision, apply a Sheets glide under the nucleus and then remove the nucleus pieces. Either way, once the incision is extended, after removing the nucleus I have to suture the wound, then do cortical removal and then open up the eye again for IOL implantation.
“In this situation, we’ve started using a technique we call IOL scaffold,” he says. “First, we bring the nucleus up above the iris. Then, we inject a three-piece foldable IOL in such a way that the IOL lies between the nucleus and the iris. The IOL prevents the nucleus from going down onto the vitreous. Now, with the phaco probe, I can emulsify the nucleus while the IOL acts like a scaffold, a temporary platform. Once the nucleus and cortex are removed, I can move the lens into the sulcus. (This technique was recently described in the journal Ophthalmology.)1
“It’s also important to know how to glue an IOL in place, in case you have no useable capsule after a rupture,” he continues. “In this situation, a surgeon might consider several other options, such as leaving the patient aphakic, implanting an anterior chamber IOL or suturing an IOL to the iris. However, our preferred alternative is glued intrascleral haptic fixation of a posterior chamber IOL.
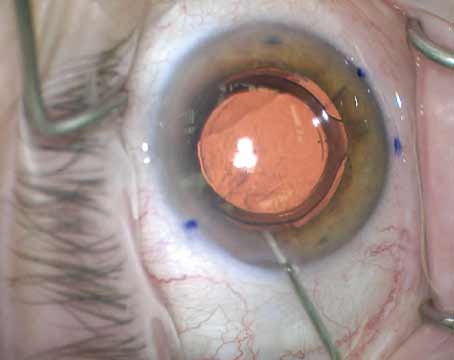
“In our technique we implant the lens, externalize the haptics, create a Scharioth intrascleral pocket, tuck the haptics in and then apply Tisseel fibrin sealant glue, which is made from human plasma,” he explains. [See example, left and facing page.] “Once this is done, the eye is sealed and the case is completed. The advantage of a glued IOL is that it’s fixed firmly in position. There’s no movement of the IOL at all—no pseudophacodonesis.”
Dr. Agarwal notes that the best way to deal with a posterior capsule rupture is to prevent it in the first place. “One of the best ways to prevent this type of complication is by using pressurized infusion,” he says. “In standard phaco surgery the fluid going into the eye is moved by gravity. Back in 1999 we developed a system that pumps air into the bottle, so that the amount of fluid that comes out is much greater—with the result that you never get any surge or have a chamber collapse that leads to posterior capsule rupture. This is called pressurized infusion, or gas-forced infusion. Today, the Bausch + Lomb Stellaris has it built in, and a modification of this system is built into the Alcon Centurion phaco machine as well.
“If you use this approach, the chamber becomes deep,” he explains. “If your rhexis is lost, you can bring the nucleus out of the bag. Because the chamber is deep, the phaco probe is away from the posterior capsule and away from the cornea; you can emulsify the nucleus immediately. I find this to be the best approach when performing phaco, and I use it in all of my patients.”
Dr. Agarwal notes that if you don’t have a Stellaris or Centurion machine, you can pressurize the infusion flow for almost no cost by purchasing an aquarium pump at a pet store, normally used to pump air into the water in a fish tank to ensure that the fish can breathe. “Take an IV tube and connect it from the aquarium pump to the IV bottle. It’s a very simple and inexpensive way to achieve this result.”
• Dropped nucleus. On rare occasions, despite the surgeon’s best efforts, the nucleus may drop to the back of the eye. “In that situation, finish up the case, unless you have a retina person onsite who can retrieve the nucleus or you yourself feel comfortable retrieving it,” says Dr. Rostov. “Do your limited anterior or pars plana vitrectomy and insert an IOL of your choice, probably a sulcus IOL in this situation. Then close up, suture the wound and call your retina person and arrange for the patient to be seen by him or her the next day. The retina person can decide when to retrieve the nucleus.”
Stay Calm and Carry On
Maybe the most important advice when something does go wrong is to monitor your own reaction. “When a complication occurs, especially a serious one, stop and take your own pulse,” suggests Dr. Rostov. “Remember that you’re still in charge. Take a deep breath and then proceed with the case. It’s important to keep any anxiety you have under control; otherwise your hand or foot can shake, and you might not be able to think clearly. Also, seeing that you’re calm will help your OR team remain calm.” REVIEW
Drs. Rostov, Agarwal and Weinstock have no financial interest in any product mentioned.
1. Narang P, Agarwal A, Kumar DA, Jacob S, Agarwal A, Agarwal A. Clinical outcomes of intraocular lens scaffold surgery: A one-year study. Ophthalmology 2013;120:12:2442-8.