Traditionally, ophthalmologists have relied on intraocular pressure measurements to guide them in assessing patients for the diagnosis of glaucoma. While IOP is certainly an important—if not the most important—risk factor for glaucoma, well-conducted studies worldwide have concluded that significant numbers of patients may have primary open-angle glaucoma without documented high IOP. Therefore, the thorough and careful optic-nerve evaluation I'll discuss in this article is essential in evaluating patients for glaucoma.
IOP: Just a Piece of the Puzzle
While IOP is a crucial component of the comprehensive eye exam, I exercise caution in focusing on it alone for the diagnosis of glaucoma. Several population-based studies suggest that one-third or more of patients diagnosed with glaucoma in the community will present without elevated IOP levels.1-3 In fact, the recently completed epidemiologic study in Tajimi City, Japan noted that IOP was 21 mmHg or less in 92 percent of patients identified with POAG.1 In a population-based cross-sectional study (a cluster-stratified random sample of 4,774 subjects), researchers identified 35 previously undiagnosed individuals with glaucoma who had visited either an optometrist or ophthalmologist or both in the previous year.4 The authors concluded that raised IOP should not be relied upon as the only initiating factor for glaucoma evaluation.
Focusing on the Nerve
After measuring the IOP and evaluating the angle (and performing gonioscopy, if indicated), I proceed to the optic-nerve exam. For optimum results, I use stereo biomicroscopy at the slit lamp. The technique is best performed with either a Hruby lens or a handheld 78- or 90-D lens. I prefer the 78-D lens because it provides a good stereo view of the optic disc with ample magnification. Some practitioners also will use the direct ophthalmoscope to supplement their disc exam. On the other hand, indirect ophthalmoscopy with either a 20- or 28-D lens is totally inadequate to perform optic-disc evaluation.
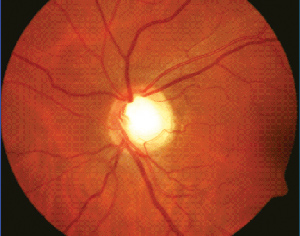
The optic-disc exam can sometimes be quite challenging because of difficulties in identifying the limits of the optic-nerve border (i.e., scleral ring). In some situations, I have found it helpful to use the green light beam on the slit lamp, since it may provide a clearer view of the scleral rim.
Here are the best ways to go about examining various aspects of the disc:
• Cup-to-disc ratio and disc rim integrity. While glaucoma optic-nerve evaluation has traditionally focused on the cup-to-disc ratio, I de-emphasize its value in glaucoma assessment. The more important measure is the extent and health of the optic disc rim tissue. In this regard, it's important to remember the ISNT (i.e., inferior, superior, nasal, temporal) rule in checking for disc rim thinning a full 360 degrees. In the normal nerve, the inferior disc rim is usually the thickest portion, followed by the superior rim, and then the nasal and temporal regions. Since glaucomatous discs tend to present with thinning and/or notching of the inferior and/or superior disc rims, they will not follow the ISNT rule.
|
In the normal population, the horizontal C/D ratio is usually larger than the vertical C/D ratio, but studies have shown that the vertical ratio increases faster in early and intermediate stages of glaucoma.5 Because of this, it may be worthwhile to focus especially on the vertical C/D ratio in the disc evaluation. I also pay particular attention to the superior and inferior disc poles, looking for notching.
I also evaluate the optic nerve's color, noting if it's pink or pale. However, focusing solely on the disc color may lead to an underestimation of the C/D ratio, since the cup region is not where the nerve tissue is situated. The tissue in the rim is what becomes thinned in eyes with glaucoma. Thus, when evaluating the significance of the C/D ratio, it is first important to measure the actual size of the optic disc. To do this, I use the 78- or 90-D lens to measure the vertical and horizontal diameters of the optic disc with the slit beam. I then multiply the diameters by correction factors (1.1 for the Volk 78 D and 1.3 for the Volk 90 D) to get an estimated disc size. I tend to define small discs as having vertical diameters less than 1.5 mm and large discs as having vertical diameters greater than 2.2 mm.
The biggest misunderstanding in glaucoma disc evaluation is that if a patient has a small C/D ratio he or she cannot have glaucoma or that a large C/D ratio means glaucoma. It's important to remember that the size of the optic disc and the size of the cup are interrelated. Thus, a normal, large disc will have a large cup, and a normal, small disc will have a small cup. This means that it is possible for a patient with a small optic disc and a small C/D ratio, such as 0.3, to have glaucomatous optic neuropathy. Still, clinicians tend to underdiagnose glaucoma in patients with small discs and overdiagnose it in patients with large discs.
To help shift the emphasis away from strictly looking at the C/D ratio, George Spaeth, MD, and his colleagues at Wills Eye Hospital in Philadelphia have advocated the concept of a Disc Damage Likelihood Scale (DDLS).6
The scale is based on the appearance of the neural retinal rim of the optic disc, corrected for the disc diameter, and also focuses on the width of the neural retinal rim and the extent of the absence of rim tissue. The DDLS has eight stages, ranging from no damage to far-advanced damage. In the clinical setting, the DDLS has been shown to be as reproducible as, or more reproducible than, the C/D ratio system in estimating the extent of disc damage in patients with glaucoma.6
|
- A C/D ratio less than 0.4 usually denotes a non-glaucomatous nerve unless the patient has an abnormally small optic disc size (which then requires further investigation);
- A C/D ratio between 0.4 and 0.8 can characterize a patient with a normal optic disc (i.e., physiologic cupping), a glaucoma suspect or someone with early to moderate glaucoma (depending on the optic disc size);
- If the C/D ratio is 0.8 or greater, consider the individual's disc as glaucomatous unless proven otherwise.
The extent of optic-disc cupping is normally symmetric between a patient's eyes, and thus, I take note of any significant asymmetry in the cupping. Greater than a 0.2-difference in C/D ratio between a patient's eyes may be an early sign of glaucoma.
The myopic optic disc has a sloped and tilted contour. In these eyes, it's often difficult to correctly evaluate the neural retinal rim, and I may rely more heavily on functional visual field testing to follow these patients.
• Peripapillary changes. I examine the surrounding region adjacent to the optic-disc border for atrophy (i.e., the alpha and beta zones) since this finding has been associated with glaucoma. Zone alpha is a peripapillary crescent region characterized by pigment irregularities in the retinal pigment epithelium. Zone beta is a chorioscleral crescent associated with thinning or absence of the retinal pigment epithelium next to the disc. If I identify these zones, the optic nerve is more likely to be glaucomatous and to be at risk for disease progression.
• Other signs. During the exam, I also look for signs of optic-disc hemorrhage, the presence of which often indicate glaucomatous optic neuropathy. The disc hemorrhages often appear as either splinter- or flame-shaped hemorrhages at the edges of the optic disc. They're rare in normal eyes and appear in approximately 4 to 7 percent of glaucomatous eyes.
Another important sign is the presence of retinal nerve fiber layer defects. These defects can be seen as distinct dark stripes (wedge-shaped aberrations in the peripapillary area) or loss of normal striations in the retina. Clinicians believe that these nerve fiber layer defects, representing the loss of specific axon bundles, may represent the most important early sign of glaucomatous optic neuropathy. In fact, clinically detectable nerve fiber layer atrophy often precedes the onset of glaucomatous field loss.8
• Differential diagnoses. To help distinguish glaucoma from other optic neuropathies, the following diagnostic clues are helpful.
Assessment of the optic nerve head's color is crucial in determining the etiology of the optic neuropathy. For example, pallor out of proportion to the extent of optic disc cupping suggests a disease of ischemic origin, such as ischemic optic neuropathy. Swelling of the nerve head could signify either an inflammatory or infectious etiology, or may be secondary to raised intracranial pressure (e.g., brain tumor). Another clue is that glaucomatous optic neuropathy tends not to present with a significant afferent pupillary defect, unless there is marked asymmetric glaucomatous damage.
It can help to check color vision in patients with suspicious optic discs. If the color vision is markedly decreased, I would suspect a neurologic etiology for the cupping.
Glaucoma is an insidious blinding disease that may present in a variety of clinical scenarios. While IOP is a major risk factor in its pathophysiology, patients can have the disease without any documented elevation in pressure. To avoid missing a diagnosis of glaucoma, a thorough and careful optic-nerve evaluation should be performed in the context of the comprehensive eye examination. REVIEW
Dr. Tsai is an associate professor of ophthalmology and director of the glaucoma division at the Harkness Eye Institute at Columbia University.
1. Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology 1992;99:1499-1504.
2. Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology 1996;103:1661-1669.
3. Iwase A, Suzuki Y, Araie M, et al. Tajimi Study Group, Japan Glaucoma Society. The prevalence of primary open-angle glaucoma in Japanese: The Tajimi Study. Ophthalmology 2004;111:1641-1648.
4. Wong EY, Keeffe JE, Rait JL, et al. Detection of undiagnosed glaucoma by eye health professionals. Ophthalmology 2004;111: 1508-1514.
5. Tsai JC, Forbes M. Medical management of glaucoma. 2nd ed. West Islip, NY: Professional Communications, 2004.
6. Spaeth GL, Henderer J, Liu C, et al. The disc damage likelihood scale: Reproducibility of a new method of estimating the amount of optic nerve damage caused by glaucoma. Trans Am Ophthalmol Soc 2002;100:181-185.
7. American Academy of Ophthalmology. Glaucoma Basic Science and Clinical Science Course. 2003-2004. San Francisco 2003.
8. Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber layer atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol 1991;109: 77-83.