With the aging population and the increasing prevalence of cardiovascular disease in the United States, it’s become more common for patients to be on some kind of antithrombotic medication such as aspirin or warfarin. These medications are vital for managing long-term comorbidities such as atrial fibrillation and venous thromboembolism, but their blood-thinning effects also increase the risk for bleeding and bleeding-related complications during glaucoma surgery.
Determining whether or not to discontinue or alter antithrombotics perioperatively is challenging. Here, I’ll discuss the risk stratification process and the effects of these drugs on glaucoma surgery.
 |
Classes of Anti-thrombotic Drugs
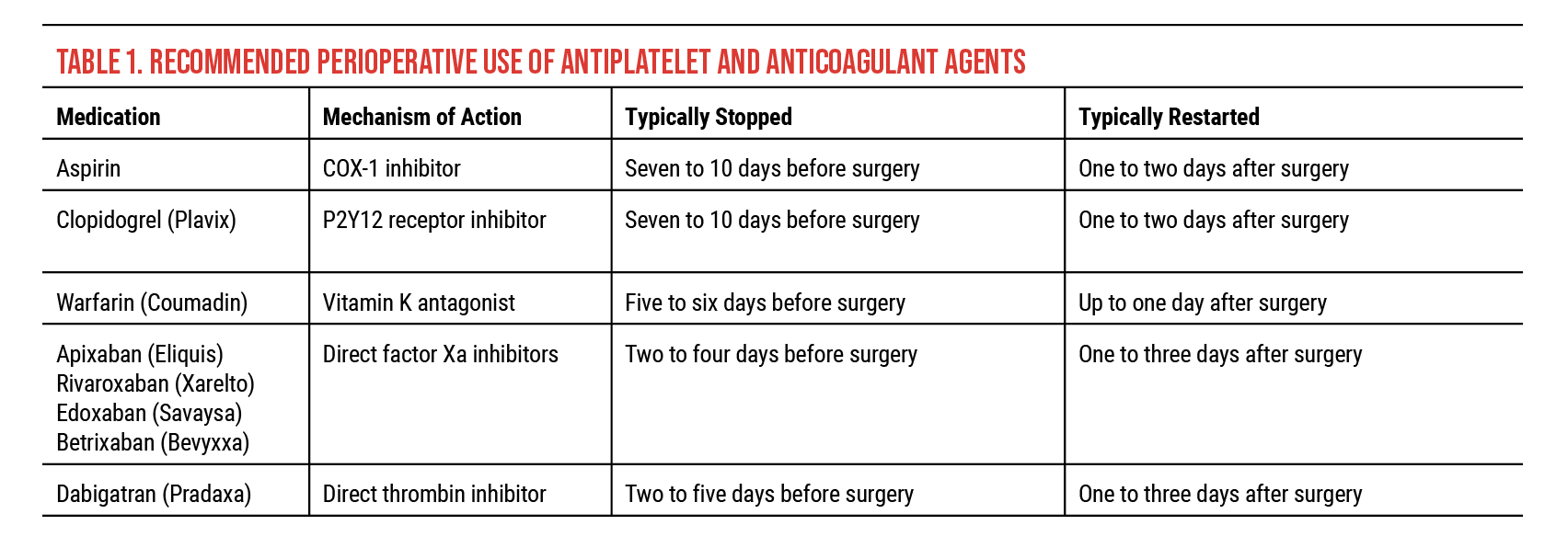
There are three classes of antithrombotic therapy (Table 1):
• Antiplatelets. Antiplatelet agents inhibit platelet function by blocking the production of a chemical involved in aggregation of platelets. Aspirin is the most commonly used antithrombotic drug, used daily in about half of adults over the age of 65. Both Aspirin and clopidogrel (Plavix) are recommended to be stopped about seven to 10 days before surgery if they are to be held. They may be restarted about one to two days after surgery. Like clopidogrel, dipyridamole, used for high-risk stroke and heart attack prevention, can be taken with aspirin.
Clopidogrel requires a functional copy of the active cytochrome P450 2C19 (CYP2C19) enzyme for efficacy, and many Chinese and Pacific Islander individuals lack this. Individuals who have two loss-of-function copies of CYP2C19 gene—approximately 2 percent of Caucasians, 4 percent of African Americans, 14 percent of Chinese, and 57 percent of Pacific Islanders—are considered CYP2C19 poor metabolizers.
• Anticoagulants. Anticoagulants inhibit the various coagulation pathways, preventing clot formation and growth. Vitamin K antagonists such as warfarin (Coumadin) block the synthesis of coagulation factors II, VII, IX and X and proteins C and S. Because of its variable half-life (20 to 60 hours), warfarin requires regular blood tests to monitor clotting time (i.e., International Normalized Ratio). Patients must remain within a fairly narrow therapeutic window, which is typically between 2 and 3 INR, depending on therapeutic indication. (Patients not on anticoagulant therapy usually have an INR of 1.) As clinicians know, fluctuations in INR are very common and can be influenced by a host of commonly used medications (e.g., NSAIDs, antifungals, antibiotics and antidepressants) and other factors such as dietary changes (including alcohol use) and illness. It’s recommended that warfarin be stopped about five to six days before surgery and restarted up to one day after surgery if it is to be held.
In outpatient settings, low molecular weight heparins are typically used for bridging warfarin therapy in high-risk patients.
• Direct oral anticoagulants. Direct oral anticoagulants are a newer class of medications consisting of direct factor Xa inhibitors, which include apixaban (Eliquis), rivaroxaban (Xarelto), edoxaban (Savaysa) and betrixaban (Bevyxxa), and direct thrombin inhibitors, such as dabigatran (Pradaxa). These medications can be stopped about two to five days before surgery and restarted up to one to three days after if they are to be held.
Direct oral anticoagulants don’t require monitoring or bridging, making them very popular in the outpatient setting. The three most commonly used agents (apixaban, rivaroxaban and dabigatran) have reversal agents. Idarucizumab (Praxibind) is approved for the reversal of dabigatran; andexanet alfa (Andexxa) is approved for the reversal of apixaban and rivaroxaban.
It’s important to ask patients why they’re using a particular type of antithrombic medication—if an antithrombotic is being used prophylactically by an individual with low thrombotic risk, temporarily discontinuing its use perioperatively may be less of an issue versus if the patient is on the medication for atrial fibrillation, for example. Patients should also be asked about the use of supplements with blood-thinning properties such as Ginkgo biloba, vitamin E or fish oil. These substances have been associated with increased bleeding risk during surgery, and it’s recommended that they be stopped before the procedure.
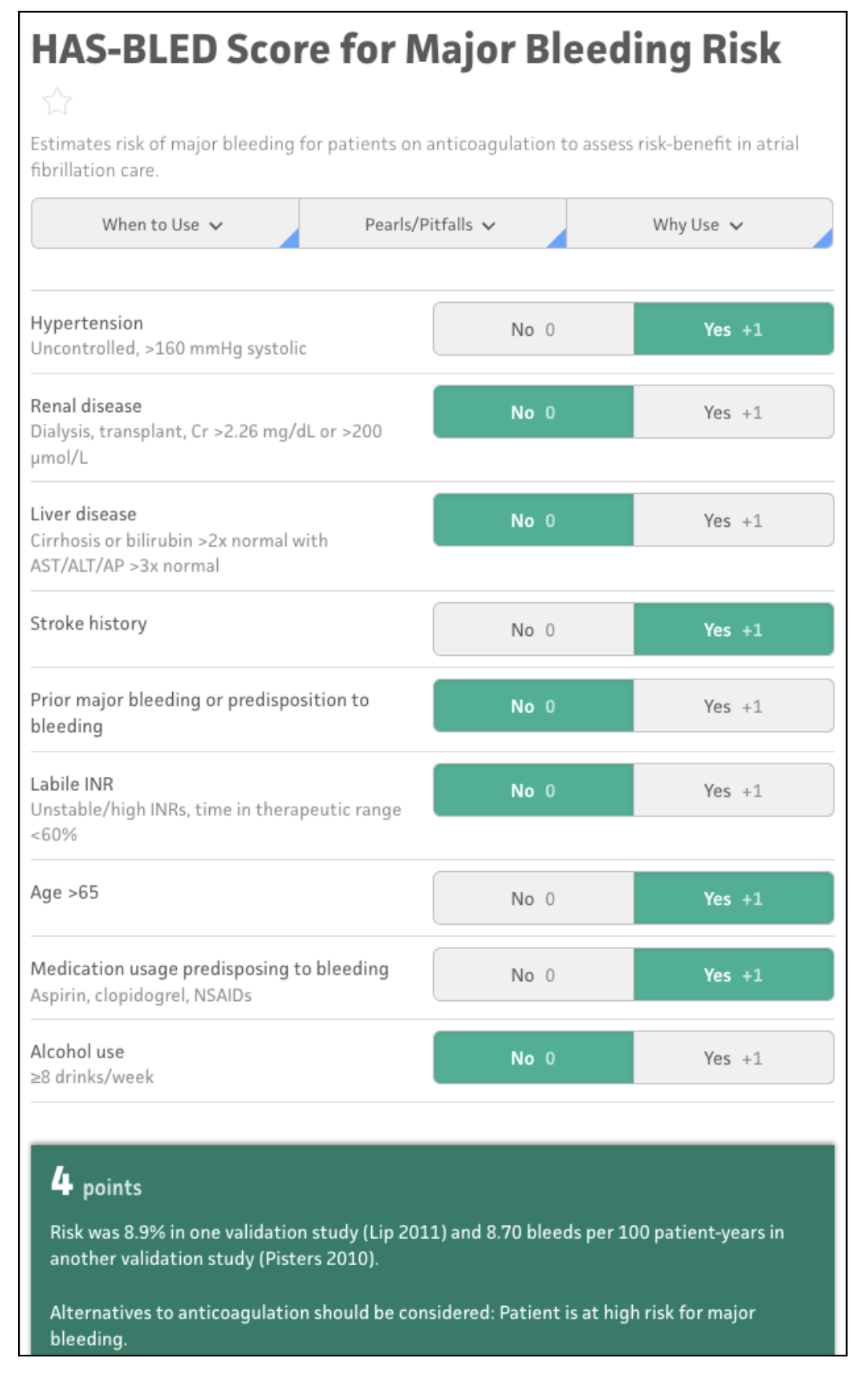 |
| Figure 1. Several risk stratification tools are available for antithrombotic management. The Hypertension, Abnormal liver/renal function, Stroke history, Bleeding predisposition, Labile INR, Elderly, Drug/alcohol usage (HAS-BLED) risk score calculator for our example patient is shown here. These free tools can help clinicians identify appropriate approaches for glaucoma surgery and perioperative medication management. (Source: mdcalc.com). |
Minimizing Bleeding Risk
Consider this case example: A 75-year-old man with open-angle glaucoma and cataracts is scheduled for cataract surgery and GATT. He’s taking Eliquis for a stroke (CVA x 2) in the setting of atrial fibrillation and high blood pressure.
The patient factor can be calculated using a host of very handy, readily available online risk-score calculators. One option is the Hypertension, Abnormal liver/renal function, Stroke history, Bleeding predisposition, Labile INR, Elderly, Drug/alcohol usage (HAS-BLED) calculator (available at www.mdcalc.com/calc/807/has-bled-score-major-bleeding-risk or as a part of the iPhone/Android app MD+Calc). This calculator estimates the risk of bleeding for patients on anticoagulation to assess the risk-benefit of anticoagulation in somebody with atrial fibrillation. According to the HAS-BLED calculator, the patient in our case example scores four points, which puts him in the high-risk category, with an 8.9-percent chance of a major bleed (Figure 1).
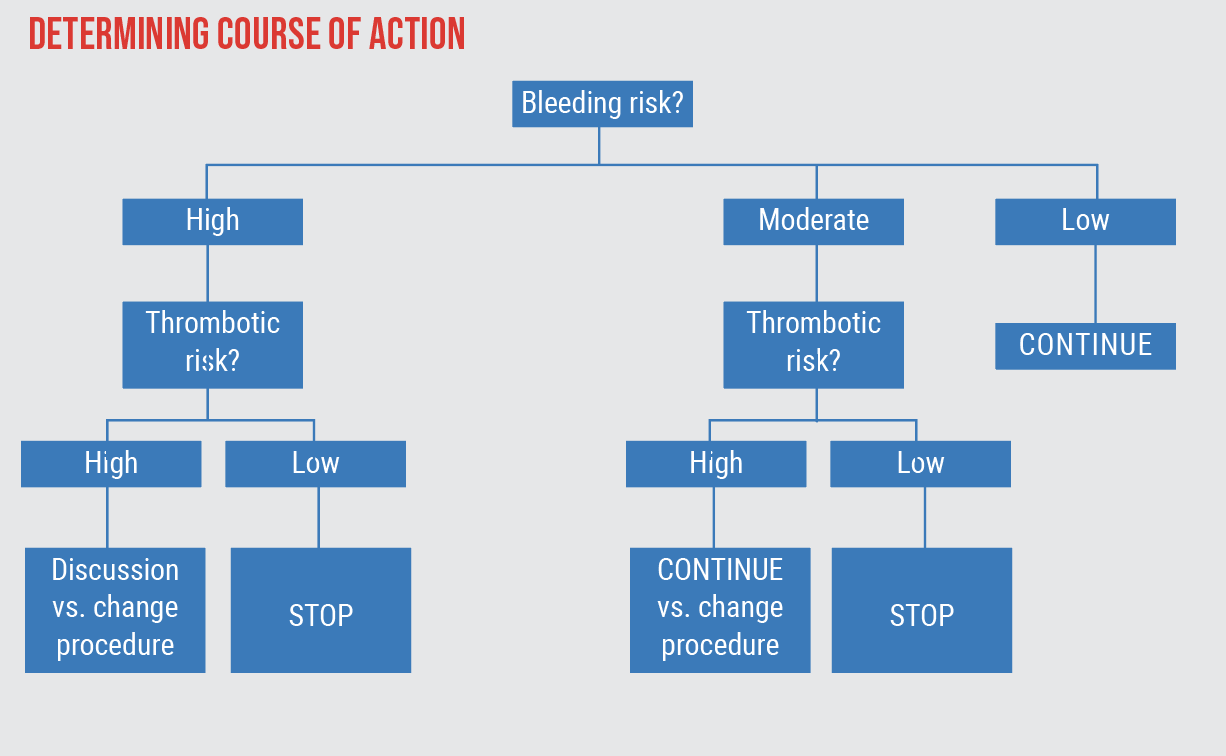 |
| Figure 2. This flowchart can be used to determine the appropriate course of action based on the planned procedures, as well as a patient’s risk for bleeding and thromboembolism. For the case example described below, the surgical factor is moderate with the proposed GATT procedure, and the HAS-BLED calculator (patient factor) shows a high bleeding risk, putting the patient on the left-hand side of the flowchart. His thromboembolism risk calculated using the CHA2DS2-VASc Score is high. This leads to the option: "Discussion vs. change procedure." It's important to discuss the risks and benefits with the patient to facilitate an informed decision. Changing the planned GATT procedure to one with lower bleeding risk may be an option. |
Minimizing Thromboembolism Risk
The second patient factor to consider is the risk for thromboembolism. Two options for calculating this risk include using the CHA2DS2-VASc Score for stroke risk in patients with atrial fibrillation (available at www.mdcalc.com/calc/801/cha2ds2-vasc-score-atrial-fibrillation-stroke-risk) or the Caprini Score, for surgical venous thromboembolism risk. The patient in our case example scored five points on the CHA2DS2-VASc Score, which is moderate-high risk.
This score can then be input into a flow chart to assess a particular patient’s risks (Figure 2). Our patient’s bleeding risk is moderate-high, so that puts him on the left-hand side of the flowchart. His thrombotic risk is also high, leading to the option: Discussing the risks and benefits with the patient to facilitate an informed decision and/or changing the planned GATT to one with lower bleeding risk.
As you might expect, there isn’t an easy solution in many cases. This patient’s situation is tricky and there’s no clear answer, but using these tools, we know a detailed and nuanced discussion with the patient is ultimately what’s necessary. It may also be necessary to involve the prescribing provider to talk about the risks of surgeries and the risks and benefits of stopping or continuing the antithrombotic agent. One thing to consider is whether GATT is still appropriate for this patient. If he’s at high risk for bleeding, perhaps a different MIGS surgery would be a better option.
What Do Colleagues Do?
While there are recommended guidelines for stopping and restarting antithrombotics in the literature, a consensus is lacking among surgeons regarding antithrombotic management for glaucoma surgery.9 Thirty percent of respondents in a U.K. study said they discontinued either warfarin or aspirin for four to seven days prior to surgery, respectively,10 whereas 80 percent of surveyed Brazilian Glaucoma Society (BGS) members said they discontinued warfarin or aspirin prior to surgery (typically seven days prior, resuming one day post-surgery).11
In the BGS study, about half of respondents reported that they experienced hemorrhagic complications that could have been related to antithrombotics, including subconjunctival hemorrhage (29.6 percent), hyphema (25.9 percent), increased postoperative bleeding (29.6 percent), and hemorrhagic choroidal detachment (7.4 percent). Ninety percent of the respondents said they referred patients to a preoperative appointment with a cardiologist or general practitioner.
Additionally, 88.5 percent of respondents said they didn’t change their usual anesthetic planning (73 percent preferred injectable anesthesia, 23 percent preferred topical anesthesia and 3.8 percent preferred general anesthesia). About 87 percent of respondents said they preferred a particular incision type in patients using antithrombotics while 13.5 percent reported that they didn’t change their technique.
At this year’s American Glaucoma Society meeting in Austin, Texas, Tejus Pradeep, MD, a resident physician at the Scheie Eye Institute, presented anonymized survey findings from our study on antithrombotic practice patterns for MIGS among AGS members.12
As might be expected, management preferences varied, depending on procedure type and surgeon preference. For example, approximately half of survey respondents preferred to defer antithrombic management to a primary care provider most or all of the time whereas the other half preferred to defer only either some of the time or never.
In summary, it’s important to pay close attention to the patient’s antithrombotic management when considering glaucoma surgery. In these situations, here are the three questions I always ask myself:
1. What is the bleeding risk of the procedure?
2. What is the bleeding risk for the patient?
3. What is the thromboembolic risk for the patient?
When appropriate, I highly recommend using the risk stratification tools available online, as well as involving the prescribing physician in decision making in antithrombotic management. Finally, a detailed conversation with the patient regarding the pros and cons of both the procedure and antithrombotic medications is critical.
Dr. Cui is an assistant professor of ophthalmology and leads the Cui Lab for glaucoma research at the Scheie Eye Institute, University of Pennsylvania in Philadelphia. She has no related financial disclosures.
Dr. Singh is a professor of ophthalmology and chief of the Glaucoma Division at Stanford University School of Medicine. He is a consultant to Alcon, Allergan, Santen, Sight Sciences, Glaukos and Ivantis. Dr. Netland is Vernah Scott Moyston Professor and Chair at the University of Virginia in Charlottesville.
1. Cobb CJ, Chakrabarti S, Chad V, Sanders R. The effect of aspirin and warfarin therapy in trabeculectomy. Eye (Lond.) 2007;21:5:598-603.
2. Law SK, Song BJ, Yu Fei, et al. Hemorrhagic complications from glaucoma surgery in patients on anticoagulation therapy or antiplatelet therapy. Am J Ophthalmol 2008;145:4:736-746.
3. Lauermann P, Klingelhofer A, Mielke D, et al. Risk factors for severe bleeding complications in glaucoma surgery and the role of antiplatelet or anticoagulant agents. Clin Ophthalmol 2022;16:1245-1254.
4. Jeganathan VSE, Ghosh S, Ruddle JB, et al. Risk factors for delayed suprachoroidal haemorrhage following glaucoma surgery. Br J Ophthalmol 2008;92:10:1393-1396.
5. Widder RA, Lappas A, Rennings C, et al. Influence of oral anticoagulation on success rates and risk of bleeding events after iStent inject implantation combined with phacoemulsification. Graefs Arch Clin Exp Ophthalmol 2020;258:11:2483-2487.
6. Shalaby WS, Patel S, Lam SS, et al. Hemorrhagic complications following trabecular bypass microstent surgery in the setting of antithrombotic therapy. J Glaucoma 2023. [Epub ahead of print January 26, 2023].
7. Pratte EL, Ramachandran M, Landreneau JR, An JA. Risk factors for hyphema following Kahook Dual Blade goniotomy combined with phacoemulsification. J Glaucoma 2022. [Epub October 28, 2022].
8. Kanda S, Fujishiro T, Omoto T, et al. The effects of antithrombotic therapy in ab inferno trabeculotomy with a spatula-shaped micro hook. PLoS One 2022;17:1:e0262548.
9. Rahman SI, Turalba A. Anticoagulation in glaucoma surgery. Semin Ophthalmol 2018;33:1:108-111.
10. Alwitry A, King AJ, Vernon SA. Anticoagulation therapy in glaucoma surgery. Graefes Arch Clin Exp Ophthalmol 2008;246:6:891-896.
11. Balbino M, Boin P, Prata TS. Perioperative management of anticoagulant users scheduled for glaucoma surgery: A survey among the Brazilian Glaucoma Society members. Arq Bras Oftalmol 2013;76:6:363-365.
12. Pradeep T, Ying G, Cui Q. Perioperative anticoagulation management patterns related to minimally invasive glaucoma surgery (MIGS). Paper presented on March 2 at the 2023 American Glaucoma Society in Austin, TX.