As doctors, we have many resources available to help our patients. However, some of those resources are underutilized simply because of a lack of awareness or an understanding of their advantages. Ocular therapies created by a compounding pharmacy may fall into this category for many of our peers.
The fact that fixed-combination drugs can be advantageous for our glaucoma patients isn’t a secret. However, we may be unaware of the value of the option of compounding pharmacies dispensing preservative-free drops (single-drug or fixed-combination) for some patients. I’ve found this option to provide significant benefits for many of my patients, with an easy implementation process.
Here, I’d like to review the many ways in which these compounded, fixed-combination drops have helped our patients achieve good outcomes with the potential benefits of lower cost, increased compliance and minimal time and effort for acquisition.
 |
Making Treatment Manageable
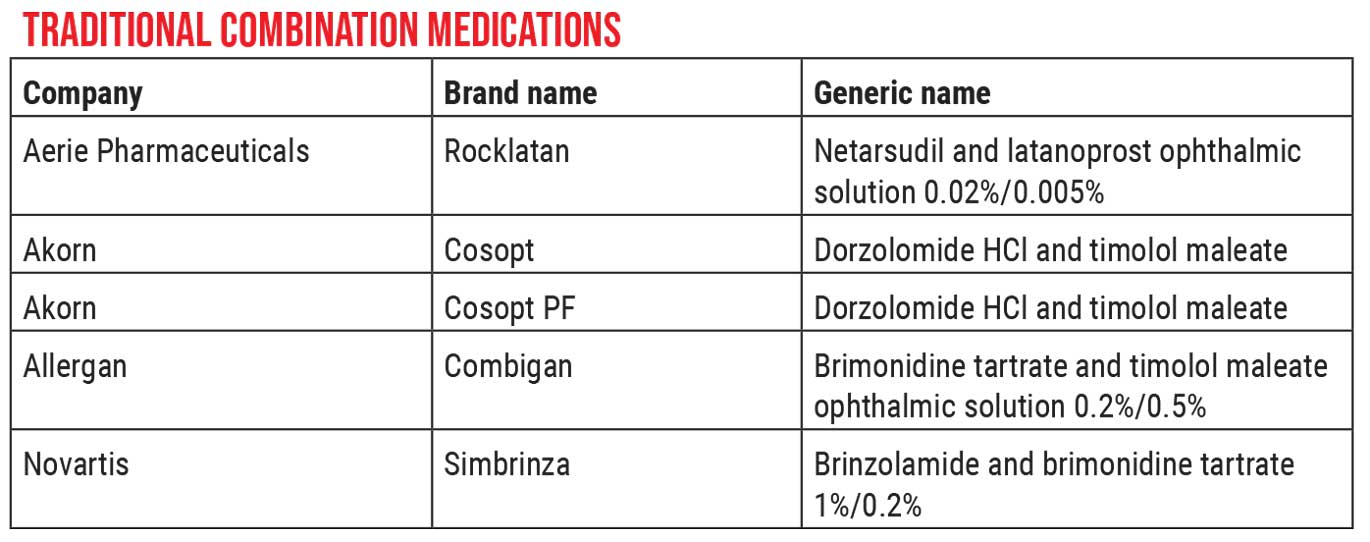
As ophthalmologists, we likely prescribe the traditional combination medications often. But many ophthalmologists seem to be unaware that compounding pharmacies can make new combinations for us, or create preservative-free versions of the familiar combination drops, all at a very reasonable cost to our patients.
I care about this topic because many of my patients have difficulty managing regimens involving multiple drops. Barriers include physical difficulty with administration and difficulty with bottle identification or following regimens. Many of our patients or their caregivers simply don’t wait the recommended time between consecutive medication applications. Furthermore, the copay burden for our patients is ever-increasing. One of my Medicare-covered patients recently told me he pays $300 a month for his glaucoma drops—and none of them were preservative-free! There has to be a better way.
The unfortunate reality is that there’s tremendous variability regarding whether traditional combination medications will be covered by a given insurance policy. They may or may not be on-formulary, and there’s a lot of paperwork at our end to get prior authorization. In contrast, the kind of “out-of-the-box” menu offered by some compounding pharmacies can be a fruitful alternative, saving both the doctor and patient time.
In the past year, I’ve been increasingly using compounding pharmacies because of the aforementioned benefits. The companies also provide excellent customer service, especially in terms of ordering and shipping them to patients in a timely manner. In addition, many of these options are surprisingly affordable. Some of the preservative-free single agents cost about $20 a month—in some cases, $20 for a two-month supply. The savings are even more notable with some of the combination drops.
Of course, combination drops are advantageous by their very nature. Benefits include:
• Reducing or eliminating the confusion associated with a complex regimen. There’s no mystery regarding why combination drops help reduce patient confusion. Patients who have multiple bottles that need to be administered at various frequencies throughout the day often use their medications incorrectly.
Studies have shown that compliance starts to decrease after adding a second agent—much less a third or fourth. For example, a patient might mistakenly take a medication twice a day that’s only supposed to be taken at bedtime. A mistake like that could actually cause an increase in the patient’s IOP. By simplifying a patient’s regimen with combination drops, we can hopefully improve adherence and potentially reduce unwanted side effects.
• Reducing side effects associated with preservatives, including ocular surface damage. Numerous studies have documented the long-term negative effects of the preservatives used in our medications, such as being toxic to the ocular surface. A recent article in Nature, published in July 2021, showed that these preservatives—the most common one being BAK—cause cytotoxic damage to both the conjunctiva and corneal epithelial cells.1 As a result, many of these patients have increasing signs and symptoms of ocular surface disease, such as dry eye and ocular irritation. Giving the patient combination drops is one way to minimize this problem. (It’s true that preservative-free formulations of some brand-name drops are currently available, but they’re either more expensive than those created by a compounding pharmacy or they require prior authorization from insurance companies.)
• Convenience. The fact that these companies mail the drops directly to the home via express shipping is very convenient for our older patients who are trying to avoid in-person interactions. (This has been especially helpful during the pandemic.) Secondly, my patients report great customer service.
• Cost savings, even compared to generics. Generic alternatives to branded medications used to be seen as a way to save patients money, but that advantage has diminished dramatically. Generic drug prices have quadrupled in the past 10 years. Combination drops made by compounding pharmacies are often significantly less expensive than generic drops.
One of my patients, currently managed on four therapeutics, is visually challenged, physically challenged, unable to get to the pharmacy and unable to manage three or four different bottles. I recently prescribed a combination drop that replaced three of the patient’s topical medications, and it only cost $40 for a three-month supply. This was a huge savings for the patient, and it was delivered to the patient’s home via Express Mail. For the patient, this was a lifesaver.
Why not prescribe the compounded preservative-free option? Many patients are uninsured or underinsured. This gives them a less-expensive alternative.
Sterility
Of course, medications must be managed somewhat differently when preservatives are omitted from the formula. The preservatives generally serve two purposes: maintaining sterility and preserving the shelf life of the medication. Preservative-free prescriptions coming from compounding pharmacies have a more limited shelf life.
In some cases, there have been sterility concerns with compounded medications such as avastin, bevacizumab and moxifloxacin, resulting in a few product recalls and advisories. But it’s important to note that these are medications that are used intraocularly. In the case of an externally applied glaucoma medication, one could say that the risk of harm to the patient—from a limited-use bottle that wasn’t thrown away at the appropriate interval—is considerably lower, but still exists.
Of course, that doesn’t change the reality that it’s of paramount importance to get patients to follow the rules. They need to understand that they can’t keep using the same unpreserved bottle for a year.
Is a generic product the same as the branded one?Doctors are often uncertain as to whether generic products are identical to the “equivalent” branded product. That’s true, in part, because we’ve all heard some ophthalmologists claim that a generic doesn’t produce the same level of efficacy.How a generic product duplicates a branded product depends on whether the medication is a solution or a suspension. Generic solutions are required to be exact copies of the original medications. That’s not the case if the generic is a suspension. For example, the majority of generic antibiotic drops are solutions, so they are exact copies. On the other hand, steroid drops are usually suspensions. This could explain why uveitis specialists often prefer the brand-name steroid drops. They sometimes find that their patients don’t respond as well to the generic; the explanation could be that a suspension isn’t an exact copy of the branded product. In any case, most compounded drops are solutions. (The bottle should state whether it’s a solution or a suspension.) —CK |
503A vs. 503B Pharmacies
There are two ways to make compounded pharmaceuticals, which is reflected in the two types of compounding pharmacies allowed by the FDA—503A pharmacies and 503B pharmacies. Both types of compounding pharmacies use bulk active and inactive substances obtained from FDA-registered manufacturers to create the combination drops.
A 503A compounding pharmacy can create a single, small batch of a compounded product for a single patient (for example, a single tube of a special dermatology cream to treat a rash). The formulation isn’t created until a physician issues a prescription for it. A 503A pharmacy isn’t allowed to make drugs in batches; its interactions are with a patient, not an office. Environmental monitoring must be performed every six months.
On the other hand, a 503B compounding pharmacy is an FDA-registered outsourcing facility that can provide bulk compounding. Its products can be created in larger batches, with or without an individual prescription. These facilities are required to maintain full compliance with current good manufacturing practices (CGMP). 503B facilities must produce multiple batches and submit them for testing and stability before a new product can be brought to market, which may result in longer relative lead times.
503B products and testing methods must be validated according to USP; similarly, all suppliers and vendors providing raw materials must be vetted. On-site inspections must be performed by quality personnel. An Environmental Monitoring program must be developed and performed— at minimum weekly, but usually more often.2,3
Of course, you can argue that there are reasons to be cautious about the use of compounding pharmaceuticals. FDA-approved, commercially marketed medications are subjected to more stringent quality standards than compounding pharmacies. Commercial manufacturers are required to prove the efficacy of the product. For example; before the FDA approves a marketed product, it has to undergo three sets of randomized controlled trials and studies of adverse events. Those studies have to demonstrate that the product is safe and effective and will last a certain length of time on the shelf.
Compounded products don’t have to go through that process. The pharmacies simply refer to the literature and use the same dosage of the key ingredients to make the product. (Furthermore, compounding pharmacies can create combinations that were never specifically FDA-approved as a product formulation.)
Nevertheless, a 503B pharmacy is FDA-registered, which means the facility has been inspected and is considered safe for creating medication in bulk. In addition, the pharmacy has to comply with certain manufacturing guidelines, and it has to test every batch for endotoxins, sterility and potency. Since compounded solutions are required to be identical to the branded medication—and nearly identical in the case of suspensions—the likelihood of a problem with lower risk medications is remote.
Evidence of Efficacy
To demonstrate the efficacy and safety of their products, some companies have performed randomized, prospective trials. These tend to be small studies, and come with the caveat that the company is performing the study. Nevertheless, they can provide some evidence (along with our own in-clinic experience) that the compounded products do what they’re expected to do.
For example, OSRX—a 503A compounding pharmacy and one of the two most widely used compounding pharmacies for ophthalmic products—conducted a proof-of-concept trial for their OMNI combination glaucoma drops, to demonstrate that these drops are as effective as individual drops used together. OSRX’s OMNI drops come in three formulas: 1) a combination of timolol and latanoprost; 2) a combination of timolol, brimonidine and dorzolamide, which they call their “A.M. Formula,”; and 3) their “P.M. Formula,” combining timolol, brimonidine, dorzolamide and latanoprost. OSRX conducted a prospective, randomized, investigator-masked multicenter study of 58 subjects with primary open-angle glaucoma who were already using at least three glaucoma drops.4 Twenty-eight of the subjects were switched to the A.M. or P.M. formula, depending on which one most closely resembled their pre-existing regimen. Over the course of 90 days, those who switched to one of the combination formulas demonstrated better IOP control and greater IOP reduction.
Admittedly, this study isn't very strong scientifically, as there are many confounding variables involved. For example, assuming that we accept the results, were they due to improved compliance or the elimination of the washout effect when multiple drops are applied too quickly in succession? The study wasn’t as clean as I’d like, but their combination drops certainly didn’t prove to be inferior to the patients’ multi-drop regimen.
My own clinical experience has largely been with the combination products produced by the other widely used compounding pharmacy: Imprimis. (I have no financial ties to either company.) I use the Imprimis drops because they offer six different combination glaucoma drops. Also, Imprimis is a 503B compounding pharmacy with the previously mentioned higher safety standards. As a result, whatever mixture of drops your patient is on, you can usually find a combination drop that contains all or most of them. Furthermore, their pricing is very simple; all of their combination drops are the same price, and preservative free.5 (There are other compounding pharmacies as well, but they don’t have as much market share in the ophthalmic space as OSRX and Imprimis.)
Getting Started, with Caution
Of course, it’s important to let patients know that I can’t guarantee that compounded drops are as safe as the branded products on the shelf in the pharmacy. The FDA tests the branded products in ways that these combination drops aren’t tested. And I counsel patients about throwing away the drops in a timely fashion. (As glaucoma specialists, we’ve all seen patients come in with an old plastic baggie full of drops that have probably been open since last year. If a patient is on preservative-free compounded medications, that’s not an option.)
If you haven’t tried this prescription alternative, but you’re interested in trying it, I’d suggest visiting the companies’ websites. Get familiar with the products they offer. Then, try them out on a couple of your tech-savvy patients and see what their experience is like.
Doing this is extremely easy from a prescribing standpoint. The Imprimis pharmacy, for example, is loaded in our electronic health records system, making it easy for me to order their drops electronically. If you have questions, you can call the companies’ customer service lines, and they’ll send you materials such as their catalogs, as well as patient advice cards, explaining how to interact with their system.
Patients to whom I offer these prescriptions constitute a fairly small segment of my practice. Of the prescriptions I write, compounded, preservative-free formulations only make up about 5 percent. But for that subset of patients, these can be a lifesaver.
Dr. Patel is an associate professor at Baylor College of Medicine and chief of ophthalmology at Ben Taub General Hospital in Houston. She reports no relevant financial ties to any product or company mentioned in this article.
Dr. Singh is a professor of ophthalmology and chief of the Glaucoma Division at Stanford University School of Medicine. Dr. Netland is Vernah Scott Moyston Professor and Chair at the University of Virginia in Charlottesville.
1. Kim DW, Shin J, Lee CK, et al. Comparison of ocular surface assessment and adherence between preserved and preservative-free latanoprost in glaucoma: A parallel-grouped randomized trial. Sci Rep 2021;11:14971:1-9.
2. Novack GD. Quality of generic ophthalmic drugs. Ocul Surf 2013;11:1:54-7.
3. Novack GD. Pipeline: Bio-generics. Ocul Surf 2006;4:3:163-5.
4. Source: osrxpharmaceuticals.com/sites/default/files/resource-files/OMNI_Brochure_02.08.21.pdf.
5. Source: imprimisrx.com/assets/Ophth_Catalog_D.pdf.