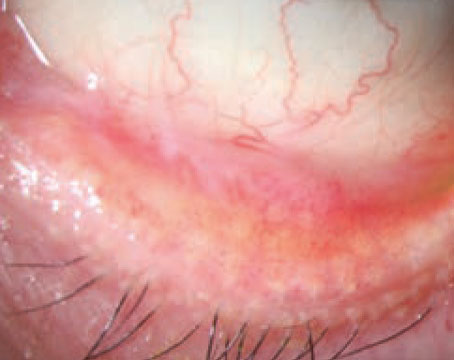
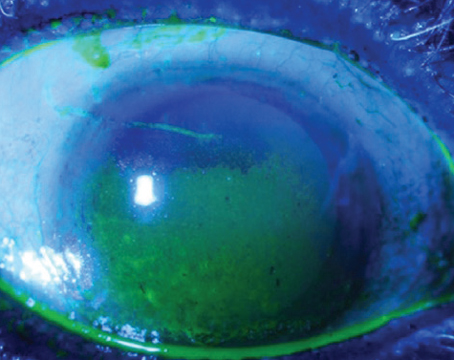
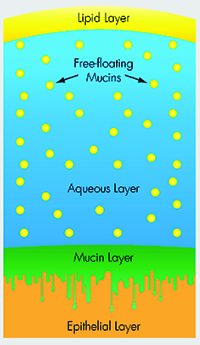
 |
| Mucin helps stabilize the tear film, and is the subject of several research studies investigating new therapies. |
A Variety of Agents
Many different therapeutic entities are under investigation for the treatment of dry eye. Besides the continued efforts to improve polymers, more targeted mechanisms are also coming into focus, from secretagogues and mucomimetics to anti-evaporatives and hormonal and nutritional supplements. Here are the different classes of drugs being investigated.
• Hyaluronic acid. Hyaluronic acid has been intensely studied in the realm of artificial tears, primarily in Europe: 0.1% solutions in saline;1 hypotonic and isotonic solutions of sodium hyaluronate;2 sodium hyaluronate 0.4% and 0.25% in combination with chondroitin sulfate;3 unpreserved, hypotonic 0.4% hyaluronic acid drops versus HPMC plus Dextran 70, 0.1% (Fermavisc);4 and 0.18% sodium hyaluronate,5,6 the last of which went the furthest down the pipeline before stalling.
Efforts to modify HA to increase its residence time on the ocular surface have provided us with improved compounds. A thiolated, carboxymethyl hyaluronic acid (CMHA-S) has been synthesized to form covalent disulfide cross-links, thus modulating the gelation of the formulation to enhance dwell time on the ocular surface. A similar formulation is already marketed worldwide for veterinary use (ReMend),7 and clinical investigations by Jade Pharmaceuticals are forthcoming in the United States. Another modified hyaluronate is being developed by Seikagaku and is in the Phase II/III stage of clinical investigation.8
Yet another tear solution containing CMC, the osmoprotective erythritol L-carnitine and glycerin, was compared to a standard HA formulation in a Phase III non-inferiority study in Europe. Compared to HA, the osmoprotective CMC-containing tear was preferred by patients, and was noninferior in terms of efficacy and safety.9
• Mucomimetic/mucogenic agents. Tamarind seed polysaccharide is a mucomimetic, mucoadhesive pseudoplastic with a structure similar to mucin 1, a transmembrane glycoprotein thought to play an essential role in ocular surface wetting. This has been evaluated alone and with HA in Europe, and is a component of Visine Intensiv 1% EDO eyedrops, available commercially in Europe.10 However, a 2014 paper demonstrated it was equivalent to an eye drop containing the barrier polymer carmellose, and as such, tamarind seed polysaccharide might end up being just an ingredient in improved tear substitute formulations rather than an active therapeutic.11
Mucomimetic agents continue to be developed in Japan, but the programs are stalled in the United States. The compounds approved in Japan are rebamipide (Otsuka/Acucela), a quinolone that enhances mucosal defense and scavenges free radicals,12,13 and diquafosol (Santen), a G-protein coupled, P2Y2 purinogenic receptor agonist.14,15 Studies of these agents primarily assessed change or improvement from baseline in signs and symptoms over the time treated. In one study designed to assess noninferiority, patients were treated for four weeks with either rebamipide 2% or 0.1% sodium hyaluronate.13 All endpoints showed noninferiority of rebamipide to hyaluronate, although rebamipide was superior for lissamine green staining.
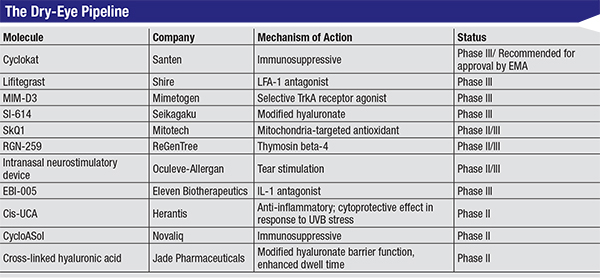 |
| Click to enlarge. |
• Autologous serum. Eyedrops composed of autologous serum are being tested on a regular basis, and publications in the past 15 years have been numerous.16-18 Twenty percent serum in saline or sodium hyaluronate has been evaluated in severe dry eye or in dry eye associated with graft-versus-host disease.16 By instilling up to 10 times daily, symptoms and signs were significantly improved. Punctal plugs may be used in conjunction with serum eyedrops to heighten their efficacy. While there is understandably not much impetus from pharmaceutical companies on this front, if an allogeneic serum eyedrop were developed, preserved and in some way formulated in a patentable fashion, patients might benefit from this technology.
• Antioxidant therapy. Scavenging free radicals is the role of every antioxidant, and these molecules are in high demand in all avenues of medicine and nutriceuticals. One promising antioxidant with a somewhat different mechanism is SkQ1, a mitochondria-specific plastoquinone-containing reactive oxygen species scavenger. In Phase II testing of 91 dry-eye subjects exposed to a controlled adverse environment challenge, improvements in central fluorescein and lissamine green staining, lid margin redness and the symptoms of discomfort, dryness and grittiness were all significant after one month of treatment with SkQ1.19 The ophthalmic formulation was approved in Russia as Visomitin in 2012 for treatment of dry eye.
• Receptor inhibition. Mimetogen is developing a dry-eye therapeutic, MIM-D3, that has an innovative mechanism of action. Conjunctival mucin gene expression and secretion, as well as goblet cell differentiation, are stimulated by nerve growth factor action at the tyrosine kinase A receptor. MIM-D3 is a partial TRKA receptor agonist that demonstrates activities similar to NGF, and also acts by potentiating suboptimal concentrations of NGF. Although it didn’t meet its pre-specified primary endpoints, after four weeks of treatment in a Phase II trial, MIM-D3 significantly improved total corneal fluorescein staining when assessed as the change from pre- to post-exposure in the CAE chamber.20 Inferior corneal fluorescein and lissamine green staining revealed improvements after 14 and 28 days of treatment. The four-week treatment with MIM-D3 also significantly improved diary-reported ocular dryness (p=0.034). In-office assessment of symptoms also showed improvements in CAE-induced stinging at day 14 (p=0.029) and at day 28 (p=0.013). Furthermore, in patients with more severe symptoms at baseline, diary-reported symptoms of dryness (p=0.015) and worst symptom scores (p=0.036) were significantly improved in the 1% MIM-D3 group, and ocular discomfort was significantly improved in the 5% MIM-D3 treatment group (p=0.014). This drug is now in Phase III development.
A thymosin beta-4 antagonist has completed Phase II development with publication of results in the CAE model. As frequently occurs, neither of the primary endpoints showed a significant difference between treatment and placebo groups; however, a number of secondary endpoints demonstrated significant treatment benefits. In a single-center study of 72 subjects treated with RGN-259 (0.1% Tβ4) for 28 days, discomfort scores in the CAE were significantly improved, as were central and superior corneal fluorescein staining.21 The newly formed company ReGenTree is planning an upcoming Phase III study in the United States and Korea that will take this drug to the next level. The company is a joint venture in the United States between RegeneRx Biopharmaceuticals, which originally developed Tβ4 for multiple indications, and the Korean company G-treeBNT.22
• Oculeve tear stimulator. An innovative intranasal device from Oculeve that stimulates tearing will continue clinical testing this year.23 This is similar to an intraoral electrostimulating device used to treat xerostomia.24 Allergan has acquired Oculeve’s device, which has shown safety and efficacy in four clinical studies in more than 200 patients. Allergan plans to conduct two additional pivotal trials prior to FDA submission, which is expected in 2016.25 This creative solution to dry eye will be interesting to watch for, and teaches us yet again to think outside of the box for dry-eye therapeutics.
The Anti-Inflammatory Pipeline
Treating inflammation has always been a mainstay for dry-eye therapy, with cyclosporin A (Restasis, Allergan) leading the way, and several other promising agents coming up fast. Here’s a look at what’s making its way to the clinic.
• Lifitegrast. In the lead in the race to approval is Shire’s lifitegrast, the lymphocyte function antigen-1 (LFA-1) antagonist and a first-in-class integrin anti-inflammatory agent specifically engineered for ophthalmic indications.
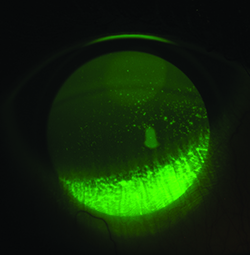 |
| In its Phase III study, lifitegrast significantly reduced total fluorescein staining. |
Lifitegrast has been the subject of three clinical trials involving b.i.d. treatment for 84 days. The first dose-response Phase II trial in 230 subjects used the CAE challenge to enrich the study population with subjects who had a modifiable, moderate degree of inferior corneal staining. Mean change from baseline to day 84 in inferior corneal staining was statistically significant (p=0.0208). Furthermore, the proportion of patients with an increase in inferior staining greater than one at day 84 compared to baseline was 16.1 percent for placebo, 3.6 percent for 1% and zero for 5% lifitegrast. The Ocular Surface Disease Index and the vision-related OSDI showed significant improvements, as did Ora scale-measured ocular discomfort (p=0.0442) in the per-protocol population. Improvements in tear production (p=0.0392) and symptoms were also seen as early as day 14. A reduction in burning/stinging was also found after 84 days of treatment when assessed as change from baseline (p=0.0496).27
The first Phase III study of lifitegrast was conducted with the same study design but with greater power due to an increased number of subjects. Again, mean change from baseline in inferior corneal staining as measured by the Ora scale was highly significant, this time with the p-value going from p=0.0208 to p=0.0007. It also significantly reduced superior and total fluorescein staining, as well as conjunctival lissamine green staining. The co-primary endpoint of OSDI symptoms wasn’t met. Secondary symptom scores for Ora-scale measured discomfort (p=0.0273) and dryness (p=0.0291) were significant, however.28
The OPUS-2 study has been completed in 718 subjects, and the company has announced success with symptom endpoints. While this study failed in the co-primary corneal fluorescein staining endpoint, symptoms won big across the board in primary (p=0.001), secondary and tertiary symptom outcomes.29
• Cyclosporine A derivatives. In recent years, researchers have attempted various changes in the formulation of cyclosporine in order to facilitate manufacturing and improve tolerability. Aqueous solutions of cyclosporine at 1% were shown to be superior to 0.5% in improving the major signs of dry-eye disease as early as 21 days after initiation of treatment.30 It will be interesting to see if anyone succeeds in getting another cyclosporine on the market either as a generic or as a new patentable entity with its structure or formulation significantly modified. Recent Phase IV studies of Restasis have shown that it significantly slows or prevents disease progression from mild to moderate or severe dry eye.31
| |
|
Novaliq in Germany is also developing a novel formulation of cyclosporine (CyclASol) that’s entering Phase II development. CyclASol is a novel, patented, non-aqueous and preservative-free formulation of cyclosporine. EyeSol is Novaliq’s proprietary drug delivery technology based on the chemically and biologically inert semi-fluorinated alkanes. These compounds have extraordinary spreading properties that optimize drug distribution on the corneal surface, and aid in the solubility of poorly water-soluble compounds such as cyclosporine. As such, CyclASol is the first clear, non-emulsion, multidose, preservative-free solution of this immunosuppressive, promising greater tolerability, less stinging and superior pharmacokinetics. Phase I results were positive and the Phase II program is under way.33
• Steroids and NSAIDs. Anti-inflammatories don’t end with cyclosporine. Steroid use has been studied, both alone and as an adjunct to cyclosporine. Ocular Therapeutix has a slow-release, biodegradable dexamethasone punctal plug that’s being investigated for both allergy and dry eye.34 Investigators have evaluated a 30-day treatment with twice-daily, low-concentration 0.1% clobetasone butyrate eyedrops in a 2% polyvinylpyrrolidone vehicle compared to vehicle alone. They found the therapy to be safe, with no intraocular pressure or fundus changes. Patients treated with just PVD vehicle showed a statistically significant improvement in symptoms and a reduction in HLA-DR expression, a dry-eye marker. Patients treated with clobetasone showed statistically significant improvements in corneal and conjunctival staining versus baseline and versus the vehicle group. The symptom scores were also significantly better compared to both baseline and vehicle, as were the HLA-DR expression and epithelial cell area. These results, both compared to baseline and to parallel vehicle control, are a powerful testament to the efficacy of a 30-day treatment of low-concentration steroid in a disease that we commonly think of as not steroid-responsive.35
In another recent report, researchers studied the effect of loteprednol etabonate 0.5% administered two weeks before initiation of cyclosporine in 118 patients. When compared to subjects treated with cyclosporine alone, the steroid group had significantly less cyclosporine-induced stinging, and more importantly, loteprednol-treated subjects had significantly better OSDI scores and Schirmer’s test scores, as well as fluorescein and lissamine staining.36 While this demonstrates a more rapid onset of effect when boosting with a safe steroid, it’s not known if the benefits of steroid pre-treatment could be maintained over time periods longer than 60 days. Nevertheless, our dry-eye subjects will certainly be happier if their improvement in signs and symptoms kicks in earlier.
In China, investigators compared the nonsteroidal anti-inflammatory 0.1% pranoprofen in a sodium hyaluronate drop to viscoelastic alone in 115 patients. Gradual improvements in symptom scores, fluorescein staining and TFBUT were shown, with statistical significance for the latter two at day 14. This anti-inflammatory was well-tolerated with 28 days of dosing.37 Fluorometholone 0.1% plus 0.1% HA was tested in clinical trials in China, comparing the combination to cyclosporine. After eight weeks of treatment, mean staining, OSDI scores, conjunctival cell density and redness were all significantly improved in both groups compared to baseline. However, fluorometholone + HA was superior to cyclosporine for mean staining at week two, OSDI scores and redness at week four and TFBUT at week eight.38 The positive effects of these various steroids for treatment of dry eye are worth exploring in creative ways with our patients, particularly when a worsening of symptoms—brought on by abrupt environmental changes, certain activities or factors such as medication change—causes our patients to fall into an acute dry-eye crisis. (See the Therapeutic Topics in the May 2015 issue of Review for a discussion of acute dry eye.)
Another anti-inflammatory on the horizon for dry eye has a unique mechanism and origin. Cis-urocanic acid is an endogenous small molecule component of human and animal skin. It’s formed in the upper layers of the skin, and is constantly present in the human body at micromolar levels, and in millimolar levels in the epidermis after exposure to sunlight. This molecule has anti-inflammatory effects due to inhibition of c-Jun N-terminal kinase (JNK) signaling; a notable effect is that activation of the mitogen-activated protein kinase (MAPK) cascade is thought to be central to dry-eye inflammatory events. The Phase I safety and pharmacokinetics of 0.5% and 2.5% cis-UCA eyedrops were recently published.39 In the EU, cis-UCA is being developed as a dermal cream for treatment of psoriasis and atopic dermatitis.40 A Phase II study has been completed and is listed on clinical trials.gov, and we look forward to hearing of its progress.41
IL-1 is a key inflammatory and immune mediator in many inflammatory diseases, and Eleven Biotherapeutics is developing an IL-1 antagonist (EBI-005) for treatment of ocular allergy and/or dry eye. The fate of Eleven’s research programs is not yet clear, but both Phase II and Phase III studies are listed on clinicaltrials.gov, and the company has completed a Phase III study in 669 patients at more than 40 clinical sites. In a press release, the dry-eye study results were described as failing to show significant efficacy in any primary or secondary endpoints, so the second Phase III study was shelved.42 The allergy indication remains the company’s focus for 2015-2016.
Potentially effective therapeutic agents deserve a rigorous clinical study program based on an understanding of corneal physiology, disease pathology and epidemiology, as well as pharmacology and pharmacokinetics. Having spent more than 20 years refining the precise operational execution of dry-eye clinical trials, and having had the opportunity to work with many therapeutic agents, it’s gratifying to the clinical achievements that finally hold the promise of greater choice for treating our dry-eye patients. REVIEW
Dr. Abelson is a clinical professor of ophthalmology at Harvard Medical School, and emeritus surgeon at the Massachusetts Eye and Ear Infirmary. Mr. Ousler is vice president of dry eye at Ora Inc. Ms. Smith is a medical writer at Ora Inc.
1. Condon PI, McEwen CG, Wright M, et al. Double blind, randomised, placebo controlled, crossover, multicentre study to determine the efficacy of a 0.1% (w/v) sodium hyaluronate solution (Fermavisc) in the treatment of dry eye syndrome. Br J Ophthalmol 1999;83:10:1121-4.
2. Papa V, Aragona P, Russo S, et al. Comparison of hypotonic and isotonic solutions containing sodium hyaluronate on the symptomatic treatment of dry eye patients. Ophthalmologica 2001;215:2:124-7.
3. Nepp J, Schauersberger J, Schild G, et al. The clinical use of viscoelastic artificial tears and sodium chloride in dry-eye syndrome. Biomaterials 2001;22:24:3305-10.
4. Lester M, Orsoni GJ, Gamba G, et al. Improvement of the ocular surface using hypotonic 0.4% hyaluronic acid drops in keratoconjunctivitis sicca. Eye (Lond). 2000;14:6:892-8.
5. Brignole F, Pisella PJ, Dupas B, et al. Efficacy and safety of 0.18% sodium hyaluronate in patients with moderate dry eye syndrome and superficial keratitis. Graefes Arch Clin Exp Ophthalmol 2005;243:6:531-8.
6. Vogel R, Crockett RS, Oden N, et al. Sodium Hyaluronate Ophthalmic Solution Study Group. Demonstration of efficacy in the treatment of dry eye disease with 0.18% sodium hyaluronate ophthalmic solution. Am J Ophthalmol 2010;149:4:594-601.
7. Williams DL, Mann BK. Efficacy of a crosslinked hyaluorinc acid-based hydrogel as a tear film supplement: A masked controlled study. PLOS One 2014;9:6:e99766.
8. https://clinicaltrials.gov/ct2/show/NCT02205840?term=Seikagaku&rank=5. Accessed 14 August 2015.
9. Baudouin C, Cochener B, Pisella PJ, et al. Randomized, phase III study comparing osmoprotective carboxymethylcellulose with sodium hyaluronate in dry eye disease. Eur J Ophthalmol 2012;22:5:751-61.
10. Jacobi C, Kruse FE, Cursiefen C. Prospective, randomized, controlled comparison of SYSTANE UD eye drops versus VISINE INTENSIV 1% EDO eye drops for the treatment of moderate dry eye. J Ocul Pharmacol Ther 2012;28:6:598-603.
11. Barabino S, Rolando M, Nardi M, Bonini S, Aragona P, Traverso CE. The effect of an artificial tear combining hyaluronic acid and tamarind seeds polysaccharide in patients with moderate dry eye syndrome: A new treatment for dry eye. Eur J Ophthalmol 2014;24:2:173-8.
12. Kinoshita S, Oshiden K, Awamura S, et al. A randomized, multicenter Phase III study comparing 2% rebamipide (OPC-12759) with 0.1% sodium hyaluronate in the treatment of dry eye. Ophthalmology 2013;120:6:1158-65.
13. Kinoshita S, Oshiden K, Awamura S, et al. Rebamipide Ophthalmic Suspension Phase III Study Group. A randomized, multicenter Phase III study comparing 2% rebamipide (OPC-12759) with 0.1% sodium hyaluronate in the treatment of dry eye. Ophthalmology 2013;120:6:1158-65.
14. Matsumoto Y, Ohashi Y, Watanabe H, et al. Diquafosol Ophthalmic Solution Phase II Study Group. Efficacy and safety of diquafosol ophthalmic solution in patients with dry eye syndrome: A Japanese Phase II clinical trial. Ophthalmology 2012;119:10:1954-60.
15. Hwang HS, Sung YM, Lee WS, et al. Additive Effect of preservative-free sodium hyaluronate 0.1% in treatment of dry eye syndrome with diquafosol 3% eye drops. Cornea 2014;33:9:935-41.
16. Ogawa Y, Okamoto S, Mori T, et al. Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone Marrow Transplant 2003;31:7:579-83.
17. López-García JS, García-Lozano I, Rivas L, Ramírez N, Raposo R, Méndez MT. Autologous serum eye drops diluted with sodium hyaluronate: Clinical and experimental comparative study. Acta Ophthalmol 2014;92:1:e22-9.
18. Semeraro F, Forbice E, Braga O, et al. Evaluation of the efficacy of 50% autologous serum eye drops in different ocular surface pathologies. Biomed Res Int. 2014;2014:826970. doi: 10.1155/2014/826970. Epub 2014 Jul 22.
19. Perekhvatova N, Petrov A, Lawrence F, Ousler GW. Evaluation of anti-oxidant SkQ1 as a treatment for the signs and symptoms of dry eye. Invest Ophthalmol Vis Sci 2015;56:4481.
20. Meerovitch K, Torkildsen G, Lonsdale J, et al. Safety and efficacy of MIM-D3 ophthalmic solutions in a randomized, placebo-controlled Phase II clinical trial in patients with dry eye. Clin Ophthalmol 2013;7:1-11.
21. Sosne G, Ousler GW. Thymosin beta 4 ophthalmic solution for dry eye: A randomized, placebo-controlled, Phase II clinical trial conducted using the controlled adverse environment (CAE) model. Clin Ophthalmol 2015;9:877-884.
22. http://regenerx.investorroom.com/2015-08-06-RegeneRx-Provides-Update-on-Clinical-Trials-in-U-S-Korea-and-China. Accessed 14 August 2015.
23. https://clinicaltrials.gov/ct2/show/record/NCT02385292?term=intranasal+device+for+tearing&rank=1. Accessed 14 August 2015.
24. Strietzel FP, Lafaurie GI, Mendoza GR, et al. Efficacy and safety of an intraoral electrostimulation device for xerostomia relief: A multicenter, randomized trial. Arthritis Rheum 2011;
63:1:180-90.
25. http://www.allergan.com/news/news/thomson-reuters/allergan-to-acquire-oculeve-dry-eye-disease-develo. Accessed 14 August 2015.
26. Murphy CJ, Bentley E, Miller PE, et al. The pharmacologic assessment of a novel lymphocyte function-associated antigen-1 antagonist (SAR 1118) for the treatment of keratoconjunctivitis sicca in dogs. Invest Ophthalmol Vis Sci 2011;52:6:3174-3180.
27. Semba CP, Torkildsen GL, Lonsdale JD, et al. A Phase II randomized, double-masked, placebo-controlled study of a novel integrin antagonist (SAR 1118) for the treatment of dry eye. Am J Ophthalmol 2012;153:1050-1060.
28. Sheppard JD, Torkildsen GL, Lonsdale JD, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease. Results of the OPUS-1 Phase III study. Ophthalmology 2014;121:475-483.
29. http://investors.shire.com/~/media/Files/S/Shire-IR/presentations-webcast/content-pdf/08-shire-mayer-latestagepipeline.pdf Accessed 25 August 2015.
30. Baiza-Durán L, Medrano-Palafox J, Hernández-Quintela E, et al. A comparative clinical trial of the efficacy of two different aqueous solutions of cyclosporine for the treatment of moderate-to-severe dry eye syndrome. Br J Ophthalmol 2010;94:10:1312-5.
31. Rao SN. Topical cyclosporine 0.05% for the prevention of dry eye disease progression. J Ocul Pharmacol Ther 2010;26:2:157-64.
32. Buggage RR, Amrane M, Ismail D, et al. The effects of Cyclokat (unpreserved 0.1% cyclosporine cationic emulsion) on corneal involvement in patients with moderate to severe dry eye disease participating in a phase III, multicenter, randomized, controlled, double-masked, clinical trial. Eur J Ophthalmol 2011;DOI:10.5301/EJO.2011.7544.
33.http://www.novaliq.de/fileadmin/Downloads/14234_NOV_Prospekt_CS_v4.1.pdf. Accessed 20 August 2015.
34.https://clinicaltrials.gov/ct2/show/NCT02468700?term=Ocular+therapeutix&rank=5. Accessed 14 August 2015.
35. Aragona P, Spinella R, Rania L, et al. Safety and efficacy of 0.1% clobetasone butyrate eyedrops in the treatment of dry eye in Sjögren’s syndrome. Eur J Ophthalmol 2013;23:3:368-76.
36. Sheppard JD, Donnenfeld ED, Holland EJ, et al. Effect of loteprednol etabonate 0.5% on initiation of dry eye treatment with topical cyclosporine 0.05%. Eye Contact Lens 2014;40:5:289-96.
37. Chen J, Dong F, Chen W, et al. Clinical efficacy of 0.1% pranoprofen in treatment of dry eye patients: A multicenter, randomized, controlled clinical trial. Chin Med J (Engl) 2014;127:13:2407-12.
38. Lin T, Gong L. Topical fluorometholone treatment for ocular dryness in patients with Sjögren’s syndrome: A randomized clinical trial in China. Medicine (Baltimore) 2015;94:7:e551.
39. Jauhonen HM, Kari E, Pylkkanen L, et al. A randomized Phase I clinical study of cis-urocanic acide eye drops in healthy adult aubjects. Acta Ophthalmol 2015;93:4:368-76.
40. Peltonen JM, Pylkkanen L, Jansen CT, et al. Three randomized phase I/IIa trials of 5% cis-urocanic acid emulsion cream in healthy adult subjects and in patients with atopic dermatitis. Acta Derm Venereol 2014;94:4:415-20.
41. https://clinicaltrials.gov/ct2/show/NCT02326090?term=cis-urocanic+acid&rank=4. Accessed 14 August 2015.
42. http://ir.elevenbio.com/releasedetail.cfm?ReleaseID=913561. Accessed 14 August 2015.