Modern imaging and the relative ease of genetic testing have enabled ophthalmologists to take great strides forward. As these advancements heighten diagnostic precision, physicians are becoming better equipped to detect novel pathology. We recently identified a unique pigmentary maculopathy in a cohort of patients who were receiving chronic pentosan polysulfate sodium therapy (Elmiron, Janssen Pharmaceuticals, Titusville, New Jersey), a popular medication for interstitial cystitis.1 Upon presentation to our clinic, these patients carried tentative diagnoses, including age-related macular degeneration and pattern dystrophy. Our subsequent investigations of this novel phenotype are suggestive of a preventable, vision-threatening medication toxicity that could masquerade as other known maculopathies. Here, we offer a clinician’s primer on what is known about this condition and how to avoid missing it in the at-risk patient.
Background
Interstitial cystitis is a chronic regional pain syndrome of the bladder and pelvis that predominately affects females, manifesting with urinary urgency and dyspareunia.2 More than 1 million people in the United States are estimated to be affected by this condition, which can also disrupt sleep and lead to emotional stress.3 There are only two FDA-approved therapies for IC: intravesical dimethyl sulfoxide and oral pentosan polysulfate sodium.4 PPS is a semi-synthetic analogue of biologic glycosaminoglycans, thought to act by binding to the bladder’s epithelial lining, regulating irritation and cellular permeability.5-9 Although it has been widely prescribed for decades, no known ocular toxicity had previously been reported.10
 |
In a 2018 report, we described unique macular pigment changes observed in six patients receiving chronic therapy for IC with oral PPS.1 Patients primarily identified symptoms of blurry vision and prolonged dark adaptation. On dilated fundus exam, these patients exhibited subtle macular pigmentary changes, yet fundus autofluorescence and near infrared reflectance imaging of the posterior pole revealed a striking pattern of abnormalities.
Despite a thorough review of medical history and comprehensive molecular testing, no known acquired or inherited etiology accounted for these findings. This series presented to us compelling evidence of a previously unrecognized medication toxicity, necessitating further investigation of causality and phenotype.
Investigation of Causality
Our subsequent investigations demonstrated that this unique maculopathy is strongly associated with chronic PPS exposure, not IC itself or its other therapies. In fact, this characteristic maculopathy has, to date, been exclusively diagnosed in patients reporting prior PPS exposure. To draw this conclusion, we conducted a retrospective, cross-sectional study at our institution to evaluate risk factors for development of the maculopathy.11 All patients of the Emory Eye Center who had been diagnosed with IC within a four-year period were included in the study.
The medical charts and pharmacy records of these patients were reviewed for documentation of exposure to IC therapies, including PPS, hydroxyzine, tricyclic antidepressants, gabapentin, pregabalin, cyclobenzaprine, methenamine, phenazopyridine and oxybutynin. Histories of hydroxychloroquine use and cigarette smoking were also included. Finally, expert reviewers masked to medication history reviewed ophthalmic images of all patients to identify cases of this unique maculopathy.
Of the 219 IC patients included in the study, 80 (36.5 percent) had prior documentation of exposure to PPS. Masked reviewers identified 14 cases of the characteristic maculopathy, each occurring among the 80 PPS-exposed patients.
No patient exhibited this maculopathy in the absence of PPS exposure. Among all potential risk factors examined, PPS exposure emerged as the sole statistically significant predictor of this maculopathy (p<0.0001). Median duration of PPS intake among affected patients was 18.3 years (range: 3 to 21.9 years). The cumulative medication exposure for this grouping of patients was 2.3 kg (range: 0.58 to 2.98 kg) across this timespan.
 |
Population at Risk
As the only FDA-approved oral agent for IC, PPS has been a mainstay of treatment for decades. Using claims data from a national U.S. insurer, we identified practice patterns suggesting hundreds of thousands of individuals have likely been exposed to the drug within the United States.
In a retrospective, matched cohort study within this large database, PPS-exposed patients were found to have a significantly increased risk of being diagnosed with a new macular disease at seven years.12
This study also suggested that chronicity of exposure plays a role; at the five-year timepoint of continuous treatment, there was a trend for increased risk, although it wasn’t statistically significant.
Clinical Features
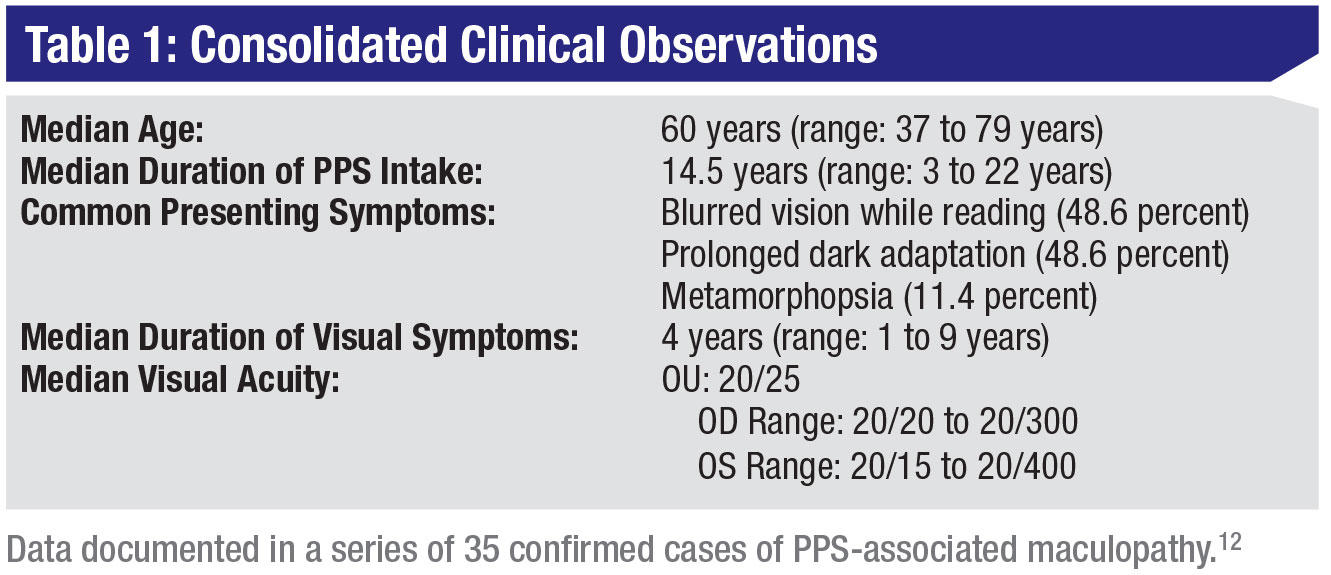
The largest case series to date analyzed 35 patients with a confirmed diagnosis of PPS-associated maculopathy across four institutions, where the median age at the time of diagnosis was 60 years (range: 37 to 79 years) (Table 1).13 The median PPS intake duration was 14.5 years (range: 3 to 22 years), at a median daily dose of 300 mg (range: 150 mg to 592 mg), consistent with recommended therapeutic regimens.9 Median cumulative intake per kilogram of body mass was 24.7 g/kg (range: 9.83 to 61.9 g/kg).
Of note: Given the recent recognition of this entity, this series likely represents a group of patients with relatively advanced disease, and it doesn’t fully capture the exposure characteristics and clinical findings of those with incipient disease.
Patients most commonly had a referral diagnosis of macular or pattern dystrophy (45.7 percent) and/or AMD (28.6 percent), and reported symptoms, including blurred vision while reading (48.6 percent), prolonged dark adaptation (48.6 percent) and metamorphopsia (11.4 percent). The median visual symptom duration was reportedly four years (range: 1 to 9 years). Visual acuity was relatively well-preserved, with median logarithm of the minimum angle of resolution (logMAR) values from both eyes measured to be 0.10 (Snellen equivalent, 20/25; OD range: 0 to 1.18, OS range: -0.12 to 1.30, p=0.93). Only 10 eyes (14.3 percent) had visual acuities lower than 20/40, of which two (2.9 percent) had visual acuities lower than 20/200. Humphrey visual field testing was typically fairly normal except in the setting of patchy atrophy of the retinal pigment epithelium.
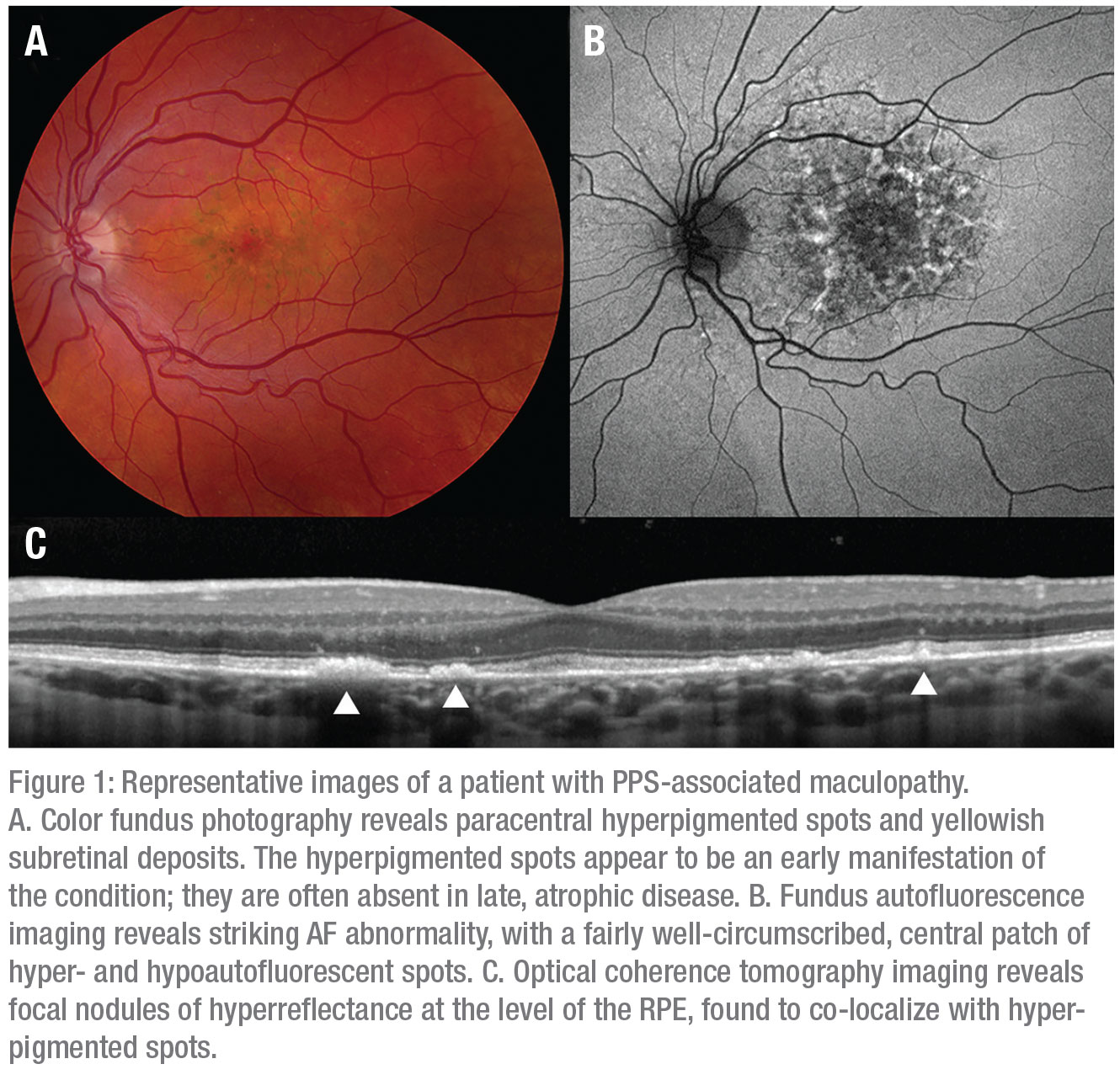
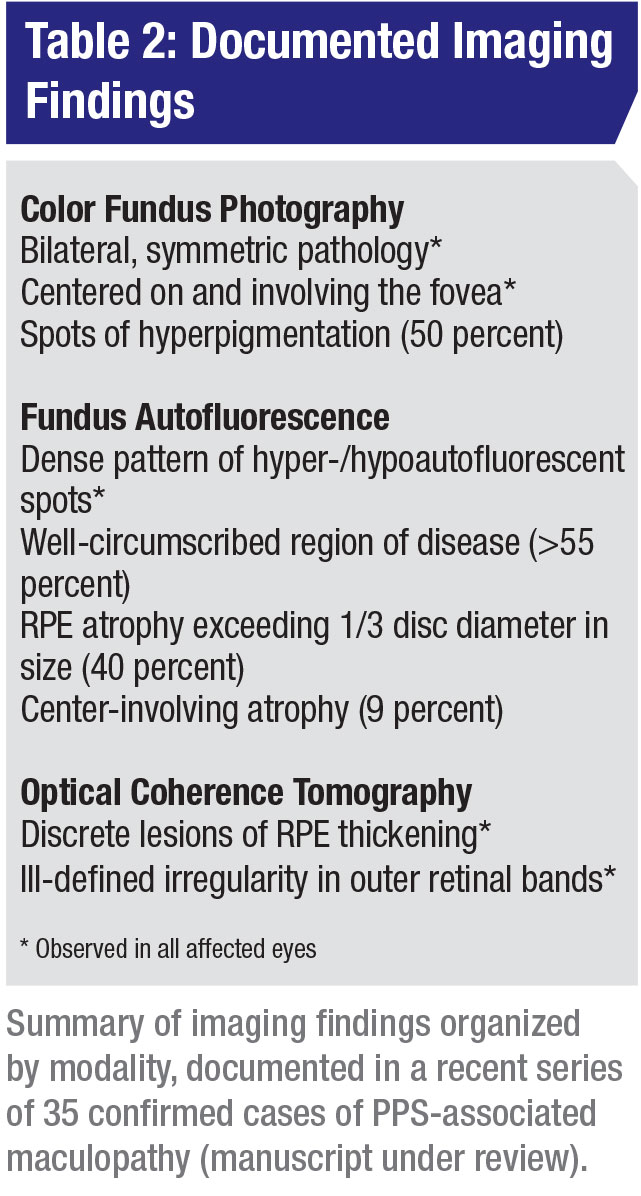
On examination, patients often had paracentral spots of hyperpigmentation accompanied by pale yellow or orange deposits (Figure 1A; Table 2). Generally, these spots initially appear in the parafoveal region and extend peripherally within the macula over time, indicating a dynamic disease process.
RPE atrophy exceeding one-third of a disc diameter was observed in 39.4 percent of cases, involving the central fovea in 9.1 percent. Spots of hyperpigmentation occurred less often in eyes with atrophy (23.1 percent) than in those without atrophy (73.7 percent, p<0.00007).
FAF (Figure 1B) imaging reveals the full extent of the fundus abnormality, which typically centers on and involves the fovea. Pathology is often characterized by a fairly well-circumscribed patch of affected retina involving the posterior pole, as was seen in 56.1 percent of patients.
However, in some cases, the FAF abnormality exhibits a more diffuse pattern, extending into the retinal periphery. Characteristic patterns within these lesions resembled densely packed hyper- and hypoautofluorescent spots and reticular hyperautofluorescent changes.
 |
OCT imaging (Figure 1C) reveals nodular thickening of the RPE that also co-localizes to the spots of hyperpigmentation observed on color fundus photography and as hyperreflectance on NIR imaging. The yellow or orange deposits observed on color fundus photography lack a clear anatomic correlate on OCT imaging, although similarly localized disruptions in the outer retinal bands may suggest that the deposits occupy the subretinal space.
All affected eyes in the cases contain regions with abnormality in the interdigitation zone, or a confluence between the interdigitation zone and ellipsoid zone bands.
Cystoid macular edema was observed in nine eyes of six patients. These cases responded well to treatments, including topical dorzolamide, and intravitreal afibercept and bevacizumab. One eye in the series developed subretinal exudation attributed to choroidal neovascularization.
Discussion
These findings bolster growing concern about a newly described medication toxicity and raise significant public health implications. Thousands of unscreened patients may be at risk. Importantly, many of these cases may have masqueraded for years as similar-appearing conditions, such as AMD and pattern dystrophy. A unique pigmentary maculopathy is strongly associated with chronic exposure to the IC drug PPS. The fundus findings in PPS-associated maculopathy are subtle, yet exhibit a distinctive clinical phenotype on multimodal imaging that’s best appreciated by using FAF. Preliminary investigations of databases indicate that many thousands of patients may be at risk.
Several possible explanations exist for this condition’s recent discovery, relative to the FDA approval of PPS in 1996.14 First, chronic exposure generally seems a prerequisite for this condition. We may only now be seeing the subset of patients who have exceeded the exposure threshold at which this disease begins to manifest. Secondly, IC patients can be complex, harboring conditions that affect multiple organ systems treated with numerous medications. This can make identifying a drug-disease association difficult.
The presenting visual symptoms for these patients are vague, and retinal changes on conventional examination are subtle. Modern retinal imaging modalities, including AF, recently adopted widely, can help detect changes. Without referral to a specialist with modern imaging instrumentation, PPS-associated maculopathy may remain undetected. Many existing cases may masquerade as similar-appearing conditions. Many patients with confirmed PPS-associated maculopathy are initially diagnosed with AMD and macular or pattern dystrophies. They may never pursue comprehensive retinal diagnostics, halting the diagnostic odyssey.15
An underlying mechanism for this condition hasn’t been fully elucidated. Fundus imaging suggests a primary insult to the RPE or RPE-photoreceptor interface. Similar to processes observed in other macular dystrophies, the RPE lesions revealed by OCT may represent dying RPE cells that have accumulated pathologic levels of byproducts from the visual cycle caused by an unknown, harmful interaction with PPS. The structural homology between PPS and interphotoreceptor matrix components may permit such an interaction. The interphotoreceptor matrix, an extracellular scaffolding that mediates the photoreceptor-RPE relationship, is composed primarily of glycosaminoglycans.16-18 PPS, a glycosaminoglycan analogue, may somehow accumulate and disrupt the structure and function of this matrix. The nodular RPE excrescences may indicate accumulation of PPS or one of its many metabolites in the RPE, as has been observed in the bladder’s urothelium.19 When radiolabeled PPS has been used to study its distribution, it has been primarily reported to deposit in the urothelium and minimally in other visceral organs.9
Recommendations
Although official recommendations for evaluating at-risk patients are premature, our institution recommends discusing the lowest necessary dose and duration of treatment with long-term PPS therapy prescribers. We suggest a baseline examination with comprehensive fundus imaging (color fundus photography, FAF imaging and OCT), followed by annual examinations with fundus imaging, starting at five years after initiation of therapy. FAF imaging is valuable for highlighting the characteristic early features of the condition.
We recommend that providers exercise caution in prescribing PPS for patients with comorbid macular disease, such as AMD, which may confer a higher susceptibility to a toxic maculopathy. Patients with elevated risk, including those with an atypical dosing regimen, those with a history of smoking or macular disease and those with comorbidities involving renal, hepatic or splenic function, may benefit from more frequent examinations.
For patients with PPS-associated maculopathy, we recommend coordination with the patient and with the prescribing physician to find an alternative regimen for IC management. Although we don’t fully understand the natural history of this condition, we advise affected patients that visual symptoms may persist and possibly worsen, even after drug cessation.
We expect to draw more conclusions about the incidence and history of CP. Investigations by our group and others will examine this unique phenotype longitudinally across multiple functional testing and imaging modalities to provide guidance regarding long-term prognosis. Ongoing animal studies will explore underlying mechanisms.
Given the emerging evidence linking PPS to macular disease, ophthalmologists have a new role in protecting patients at risk for this vision-threatening condition. Many of us may unknowingly follow affected patients. Many patients harboring this condition may either be undetected or misdiagnosed with similar-appearing conditions.
As in cases involving hydroxychloroquine and other drug-associated maculopathies, the use of modern fundus imaging techniques will help identify affected patients. We look forward to ongoing research developments that will improve our understanding of the pathobiology and prognosis of this unique maculopathy. REVIEW
Dr. Hanif is a recent graduate of Emory University School of Medicine in Atlanta. Dr. Jain practices as an assistant professor in the Division of Vitreoretinal Surgery and Disease at the Emory Eye Center.
Please direct correspondence to Nieraj Jain, MD, 1365B Clifton Road, N.E., Suite 2400, Atlanta, GA 30322.
Funding for this work has been provided by a VitreoRetinal Surgery a Foundation Research Grant (AH), Foundation Fighting Blindness Career Development Award CD-C-0918-0748-EEC (NJ) and a National Institutes of Health Core Grant P30 EY006360 (NJ).
The authors have no potential conflicts of interest to disclose.
1. Pearce WA, Chen R, Jain N. Pigmentary maculopathy associated with chronic exposure to pentosan polysulfate sodium. Ophthalmology 2018;125:11:1793-1802.
2. McLennan MT. Interstitial cystitis: Epidemiology, pathophysiology, and clinical presentation. Obstet Gynecol Clin North Am. 2014;41:3:385-95.
3. Hanno PM, Erickson D, Moldwin R, Faraday MM. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Jurol 2015;193:5:1545-1553.
4. Nickel JC, Moldwin R. FDA BRUDAC 2018 Criteria for interstitial cystitis/bladder pain syndrome clinical trials: Future direction for research. J Urol. 2018; 200:1:39-42.
5. Frileux C. [Thrombocid: a new synthetic anticoagulant]. Presse Med. 1951;59:8:159.
6. Meng E, Hsu YC, Chuang YC. Advances in intravesical therapy for bladder pain syndrome (BPS)/interstitial cystitis (IC). Low urin tract symptoms 2018;10:1:3-11.
7. Giusto LL, Zahner PM, Shoskes DA. An evaluation of the pharmacotherapy for interstitial cystitis. Expert Opin Pharmacother. 2018;19:10:1097-1108.
8. Chen P, Yuan Y, Zhang T, et alXu B, Gao Q, Guan T. Pentosan polysulfate ameliorates apoptosis and inflammation by suppressing activation of the p38 MAPK pathway in high glucose-treated HK2 cells. Int J Mol Med. 2018;41:2:908-914.
9. Elmiron (R) [Package Insert]. Titusville, NJ: Jannssen Pharmaceuticals, 2012.
10. Al-Zahrani AA, Gajewski JB. Long-term efficacy and tolerability of pentosan polysulphate sodium in the treatment of bladder pain syndrome. Can Urol Assoc J. 2011;5:2:113-118.
11. Hanif AM, Shah R, Yan J, et al. Strength of association between pentosan polysulfate and a novel maculopathy. Ophthalmology 2019 Apr 18 [Epub ahead of print].
12. Li AL, Jain N, Yu Y, VanderBeek BL. Association of macular disease with long-term use of pentosan polysulfate sodium: findings from a large U.S. national insurance database. May 1, 2019. Association for Research in Vision and Ophthalmology. Vancouver, BC.
13. Hanif AM, Taylor S, Armenti S, et al. Expanded clinical spectrum of pentosan polysulfatesodium-associated pigmentary maculopathy. The Association for Research in Vision and Ophthalmology; April 30, 2019. Vancouver, B.C.
14. Dell JR, Parsons CL. Multimodal therapy for interstitial cystitis. J Reprod Med. 2004;49:3 Suppl:243-52.
15. Hanif AM, Yan J, Jain N. Pattern dystrophy: an imprecise diagnosis in the age of precision medicine. International ophthalmology clinics 20109;59:1:173-194.
16. Clark SJ, Keenan TD, Fielder HL, et al. Mapping the differential distribution of glycosaminoglycans in the adult human retina, choroid, and sclera. Invest Ophtdhalmol Vis Sci. 2011;52:9:6511-21.
17. Brandl C, Schulz HL, Charbel Issa P, et al. Mutations in the genes for interphotoreceptor matrix proteoglycans, IMPG1 and IMPG2, in patients with vitelliform macular lesions. Genes (Basel) 23;8:7.
18. Ishikawa M, Sawada Y, Yoshitomi T. Structure and function of the interphotoreceptor matrix surrounding retinal photoreceptor cells. Exp Eye Res. 2015;133:3-18.
19. Pantazopoulos D, Karagiannakos P, Sofras F, et al. Effect of drugs on crystal adhesion to injured urothelium. Urology. 1990;36:3:255-9.



