The Scope of the Problem
Keratoconjunctivitis sicca or dry-eye disease is a condition affecting nearly 5 million Americans over the age of 50, the main causes of which are manifold. Dry-eye disease has often been oversimplified as being characterized by a deficiency of the tear film1 that leads to ocular burning, stinging, foreign body sensation or blurred vision, since the use of replacement tear substitutes does account for the majority of the recommended therapies. It can vary in severity from mild discomfort to a more severe loss of visual function, pain and light sensitivity. The collected combination of one or more of these symptoms leads to a potentially profound lowering of quality of life. While Restasis is helpful in some patients by virtue of its anti-inflammatory effect, tear substitutes offer only periodic, short-lived relief.
Although we have learned a great deal about the pathophysiology of dry eye, its treatment still challenges us, and what we perhaps considered to be a simple shortage of ordinary tears is in fact a far more complex issue. Despite efforts to develop secretagogues, anti-inflammatories and other tear modulators, we still—surprisingly—rely on the symptomatic relief offered by tear substitutes as one of the primary means of dry-eye management.
Artificial Tears
| Over-the-Counter Tear Options | |
| Artificial Tears (Akorn) Refresh Celluvisc (Allergan) Clear Eyes CLR (Prestige Brands Holdings Inc.) GenTeal (Alcon) Hypotears (Alcon Novartis) Isopto Tears (Alcon) Lacri-Lube S.O.P. (Allergan) | Oasis Tears (Oasis Medical) Opti-Free (Alcon) Optive (Allergan) Refresh Tears (Allergan) Soothe (Bausch + Lomb) Systane (Alcon) TheraTears (Akorn) Visine Tears (J&J) |
The first tear substitutes in modern times were saline-based isotonic or hypotonic solutions. These, unfortunately, had poor distribution over the ocular surface and low retention time, producing only momentary relief and requiring very frequent administration. Another group of tear substitutes included natural or synthetic polymers like methylcellulose derivatives, polyethylene glycol and polyvinyl alcohol which, due to their higher viscosity, contributed to a better retention time. A third group of tear substitutes included lipid emulsions that increased retention time, reduced evaporation from the ocular surface and improved the stability of the lipid layer.
Ingredients in artificial tear products have been shown to have different effects on comfort and on signs of dry eye. These effects, however, are mostly short-lived, providing relief from discomfort for a mere 10 or 20 minutes, and therefore requiring repeated instillations. One way to address this issue is by modulating drop viscosity, as a product with a higher viscosity will tend to have a longer retention time on the ocular surface. Conversely, if it’s too viscous, a drop of it can cause blurring or lid-caking. A measure of the importance of this issue is reflected in the hundreds of patents issued in recent years covering manufacturing of tear substitutes within a specific viscosity range or with other specific physical properties relating to viscosity.2 Refinements of drop formulations with the aim of achieving optimal viscosity are ongoing. Of these, hyaluronic acid-based products have been a focus in many dry-eye trials, and there’s reason to believe they might be the best of the viscous additives. These should soon to be available by prescription or over-the-counter.
Let’s take a deeper look into hyaluronic acid and its viscoelastic properties, and see how these properties can provide for positive outcomes in dry-eye treatment.
Hyaluronic Acid
Hyaluronic acid is a naturally occurring polysaccharide that’s ubiquitous in humans and other animals. What makes HA such an ideal lubricant is that it is amphipathic, with both hydrophobic and hydrophilic traits. This amphipathic nature yields a unique biological behavior. Hundreds of monosaccharide rings are linked to form a planar hydrophobic surface, acting like a long-chained fat molecule—energetically happiest when stacked and away from aqueous environments. At the same time, the sugars have acid side-chains projecting laterally, creating a hydrophilic edge stabilized by a crowd of adoring water molecules. The HA monomers are like ribbons in which the face of the ribbon is the hydrophobic domain, and its edges are the hydrophilic domain. This combination of features gives it its viscoelasticity, its capacity to retain water and its ability to occupy a large volume at a relatively low concentration. They also allow it to act as a selective permeability barrier where small molecules can diffuse through it at faster rates than larger molecules.3
Therapeutic uses of HA abound in many branches of science and medicine. HA is used for intra-articular injections to replace synovial fluid in patients with osteoarthritis, to control post-surgical adhesions and prevent scar formation, and to promote wound healing. It’s better-tolerated than collagen injections when cross-linked preparations are injected into the skin to aesthetically treat wrinkles.3 Three-dimensional tissue culture using HA-containing scaffolds enhances tissue growth and differentiation and is now the foundation of many types of tissue engineering and drug-delivery applications.4,5 HA preparations are of course useful in ophthalmology due to the viscosity of HA, a property that helps conserve spatial orientations of ocular tissues during surgery.3 These unique viscoelastic characteristics, as well as its tolerability and biodegradability, allow for numerous potential applications, including the use of HA as an active ingredient in tear substitutes. But is it inert or is it actually pharmacologically active?
The viscoelasticity of HA-based formulations allows for better tear stability, longer retention time and improvement of dry-eye symptoms, all of which have made it beneficial in the management of dry eye. A review of the positive outcomes brought on by different artificial tears showed increasing improvements in the percentage change in rose bengal scores of 25.9 ±18.4 with saline, 38 ±20.7 with polyacrylic acid and 41.8 ±16.3 with hyaluronic acid products.6 A comparative study revealed that HA formulations provided superior ocular comfort relative to other viscous comfort agents like hydroxypropyl methylcellulose and carboxymethyl cellulose by enhancing tear-film stability, retaining tear volume and lubricating the ocular surface.7 In a separate study, sodium hyaluronate was well-tolerated and improved moderate dry-eye symptoms more rapidly than carboxymethyl cellulose-based artificial tear formulations.8
The 0.1% concentration of HA has been the most extensively studied in artificial tears. Patients treated with 0.1% HA for four weeks had statistically significant relief from burning and improved rose bengal staining compared to those treated with 1.4% polyvinyl alcohol in a crossover study.9 A 28-day treatment with 0.1% HA solution was also reported to reduce epithelial cell damage assessed by rose bengal staining, to improve Schirmer’s scores and to decrease ocular burning and grittiness.10 Instillation of 0.18% HA for periods of seven or 14 days was found to improve lissamine green staining and a global symptom frequency score.11 Long-term use of 0.15% sodium hyaluronate-containing artificial tears has been shown to reduce ocular surface damage in dry-eye patients, with no treatment-related adverse events.12 Recently, HA has been used as a combination product, or dual polymer (in conjunction with hydroxypropyl guar, a mucomimetic polymer). A study from Alcon Research showed that formulations combining HA and hydroxypropyl guar could provide synergistic benefits in improving ocular surface hydration and decreasing friction.13 These HA products appear to function as more than just ocular lubricants, since they also exert pharmacological effects on the ocular surface.
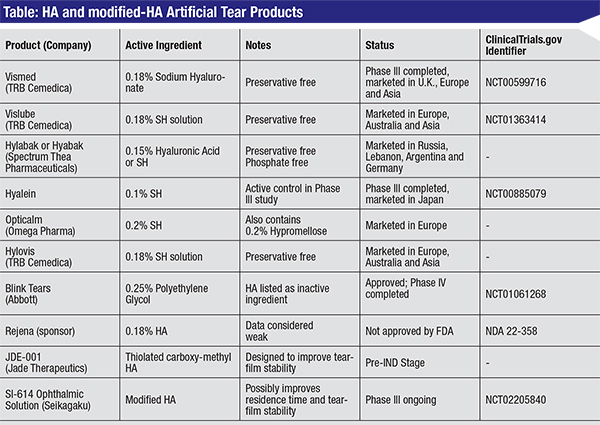 |
| (Click table to enlarge.) |
Bionic Hyaluronic?
There is an unmet medical need in the United States for next-generation HA products that can outperform the available artificial tear options in terms of increasing ocular residence time, decreasing dosing frequency, lubricating and improving the tear film, and alleviating symptoms associated with acute and chronic dry eye. While clinical trials have provided evidence that HA is superior to other polymers as a lubricant, there’s still a requirement for q.i.d. (or more frequent) dosing and much room for improvement. There is extensive literature on chemical modification of HA that may point the way for such improvements.
Pharmaceutical companies have aimed to chemically modify HA to improve its properties, thus offering superior formulations for multiple indications. The primary chemical strategy used to modify HA is the cross-link, either by direct reaction of side chains or through the addition of spacer arms, which can then form stabilizing links between HA molecules.5
One such cross-linked HA, carboxymethyl hyaluronic acid, is the active ingredient globally marketed for veterinary applications as ReMend (BayerDVM), is sold in more than 400 clinics. While piggybacking on the extensive amount of HA data already generated by others, Jade Therapeutics is in the process of bringing a formulation with this active, modified HA to human clinical trials. This lightly cross-linked eye-drop formulation is designed to modulate the gelation of the solution, thereby improving residence time and tear-film stability as compared to currently marketed products.
Another HA product is SI-614, under development by Seikagaku, a pioneer in glycoscience research since 1950. This compound has shown promise in several clinical studies, and is currently in Phase III clinical trials.
Chemically modified HA tear substitutes (shown in the table, p. 106) may represent more than an incremental improvement over the previously used artificial tear formulations, and can be viewed as a long-acting or sustained-release therapy. Rather than supplementing the existing tear film as current artificial tear formulations do, modified HA solutions are designed to be genuine treatments with significant therapeutic value.
Assessment of HA Products
With the aid of stains and other tests, we can fairly accurately assess how well a tear substitute is doing its job. The Schirmer’s test has been used for decades as a rapid and telling, albeit variable, assessment of aqueous tear production. This test would not be expected to be altered with tear substitute therapy. However, with long-term, disease-modifying treatments, it could demonstrate improved wetting. Tear-film breakup time measures instead the evaporation of the tear film, and is an assessment of evaporative dry eye due to mucin or lipid tear deficiencies. TFBUT should improve with tear substitute use, particularly if the product has an ingredient such as modified HA to increase wetting. The clinician routinely uses rose bengal, lissamine green and fluorescein dyes to stain ocular surfaces prior to examination, though lissamine green has largely supplanted rose bengal due to the potential for intolerance of the latter. While rose bengal and lissamine green preferentially stain damaged conjunctival tissues, fluorescein concentrates in areas of cell-cell junction disruption on the cornea.14
There are other more experimental tests that we frequently use to test the performance of an artificial tear product. Various measures based on the blink, including rate; inter-blink interval; patterns of blink; and partial, complete and extended blinks, can be used as surrogates for ocular discomfort and in some tests of visual function.
The ocular protection index and the interval visual acuity decay test assess very different aspects of ocular surface health with the aid of blink measures. We know that optimal blink rates enable the maintenance of a stable tear film, thus ensuring good ocular surface tissue health, while low blink rates and incomplete blinking lead to excess tear evaporation and dry eye. Ocular surface protection depends on the patient’s TFBUT matching or exceeding his or her IBI, or inter-blink interval.
The ocular protection index, which is the ratio of TFBUT over IBI, gives us a rapid snapshot of ocular surface vulnerability. If the OPI score is less than one, then a patient’s cornea is at risk of exposure; if the OPI equals or is greater than one, it is not.15 Another newer metric, OPI 2.0, measures blink rate and TFBUT simultaneously in real time.16,17 The IVAD test uses a standardized task for all patients before and after treatment, to evaluate the decay of visual acuity in the time between blinks.18 These more sophisticated measures enable investigators to establish an accurate comparative assessment of the effects of dry-eye treatments on the ocular surface and on visual function. Furthermore, using these tests in conjunction with the Controlled Adverse Environment heightens the magnitude of change from baseline and gives us a greater chance of seeing differences between vehicle and active test compounds, or between two tear substitutes.19
An adequate assessment of artificial tear products enables patients to be paired with formulations most suited to them. The future looks bright and artificial tear products with modified HA appear to have demonstrable clinical effectiveness. The use of these new products will mark the beginning of a new era for tear substitutes, an era in which we begin prescribing the best of these long-acting formulations for patients when “nature’s lotion” fails them. REVIEW
Dr. Abelson is a clinical professor of ophthalmology at Harvard Medical School, and emeritus surgeon at the Massachusetts Eye and Ear Infirmary. Mr. Ousler is vice president of dry eye at Ora Inc. Ms. Smith and Dr. Santanam are medical writers at Ora Inc.
1. Pflugfelder SC. Prevalence, burden, and pharmacoeconomics of dry eye disease. Am J Manag Care 2008;14:3:S102-6.
2. Janoria KG, Hariharan S, Dasari CR, Mitra AK. Recent Patents and Advances in Ophthalmic Drug Delivery. Recent Patents on Drug Delivery & Formulation 2007; 1:2: 161-170.
3. Robert L. Hyaluronan, a truly “youthful” polysaccharide. Its medical applications. Pathol Biol (Paris) 2015;63:1:32-34.
4. Collins MN, Birkinshaw C. Hyaluronic acid based scaffolds for tissue engineering--a review. Carbohydr Polym 2013;92:2:1262-79.
5. Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater 2011;23:12:41-56.
6. Doughty MJ, Glavin S. Efficacy of different dry eye treatments with artificial tears or ocular lubricants: A systematic review. Ophthalmic Physiol Opt 2009;29:6:573-83.
7. White CJ, Thomas CR, Byrne ME. Bringing comfort to the masses: A novel evaluation of comfort agent solution properties. Cont Lens Anterior Eye 2014;37:2:81-91.
8. Brignole F, Pisella PJ, Dupas B, et al. Efficacy and safety of 0.18% sodium hyaluronate in patients with moderate dry eye syndrome and superficial keratitis. Graefes Arch Clin Exp Ophthalmol 2005;243:6:531-8.
9. McDonald CC, Kaye SB, Figueiredo FC, et al., A randomised, crossover, multicentre study to compare the performance of 0.1% (w/v) sodium hyaluronate with 1.4% (w/v) polyvinyl alcohol in the alleviation of symptoms associated with dry eye syndrome. Eye (Lond) 2002;16:5:601-7.
10. Condon PI, McEwen CG, Wright M, et al. Double-blind, randomised, placebo-controlled, crossover, multicentre study to determine the efficacy of a 0.1% (w/v) sodium hyaluronate solution (Fermavisc) in the treatment of dry eye syndrome. Br J Ophthalmol, 1999;83:10:1121-4.
11. Vogel R, Crockett RS, Oden N, et al. Demonstration of efficacy in the treatment of dry eye disease with 0.18% sodium hyaluronate ophthalmic solution (vismed, rejena). Am J Ophthalmol 2010;149:4:594-601.
12. Aragona P, Papa V, Micali A, et al. Long-term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye. Br J Ophthalmol 2002;86:2:181-4.
13. Rangarajan R, Kraybill B, Ogundele A, Ketelson HA. Effects of a hyaluronic acid/hydroxypropyl guar artificial tear solution on protection, recovery, and lubricity in models of corneal epithelium. J Ocul Pharmacol Ther 2015;31:8:491-7.
14. Feenstra RP, Tseng SC. Comparison of fluorescein and rose bengal staining. Ophthalmology 1992;99:4:605-17.
15. Ousler GW 3rd, Hagberg KW, Schindelar M, et al. The ocular protection index. Cornea 2008;27;5:509-13.
16. Abelson R, Lane KJ, Rodriguez J, et al. Validation and verification of the OPI 2.0 System. Clin Ophthalmol 2012;6:613-22.
17. Abelson R, Lane KJ, Rodriguez J, et al. A single-center study evaluating the effect of the controlled adverse environment (CAE) model on tear film stability. Clin Ophthalmol 2012;6:1865-72.
18. Torkildsen, G. The effects of lubricant eye drops on visual function as measured by the inter-blink interval visual acuity decay test. Clin Ophthalmol 2009;3:501-6.
19. Gonzalez-Garcia MJ, González-Sáiz A, de la Fuente B, et al. Exposure to a controlled adverse environment impairs the ocular surface of subjects with minimally symptomatic dry eye. Invest Ophthalmol Vis Sci 2007;48:9:4026-32.



