Dry eye is a multifactorial disease that affects the quality and
quantity of tears and alters the ocular surface. Patients may experience
varying degrees of discomfort, including symptoms such as ocular
burning, stinging, grittiness, foreign body sensation, sensitivity to
light and blurriness.1 Signs such as keratitis are often noted to a
greater or lesser degree. Consequently, a battery of standard dry-eye
tests should be employed to properly diagnose and classify your
patients. In this month’s column, we’ll discuss the multitude of
dry-eye diagnostic tests available to ophthalmologists and how they can
assist in properly diagnosing and treating dry eye.
Beginning the Diagnosis
Dry eye’s growing prevalence and influence on everyday life is difficult
to ignore, as the polycyclic aromatic hydrocarbons in the urban
environment tend to destabilize the ocular surface by altering the
intercellular junctional complexes. This is why it’s important that
both patients and clinicians be acutely aware of dry eye’s signs and
symptoms. These symptoms are subjective and may be elicited through the
use of standard grading scales, symptom questionnaires and patient
interviews.
 Patient History
Patient History
Dry eye can develop in association with a number of autoimmune diseases, such as systemic lupus and rheumatoid arthritis, or be brought on and exacerbated by certain environmental and lifestyle adaptations. Careful examination and utilization of proper patient questioning and taking a detailed history can lead to a focused diagnosis of dry eye and ultimately a more rewarding treatment plan.
Patients may not be aware that they have dry eye, but may verbalize their symptoms in ways that can point you toward a diagnosis. Many patients notice changes in their symptoms at different times during the day, often noting changes in severity while driving at night or engaging in extended visual tasking activities such as watching television or using the computer. It’s also important to take note of any prescription medications your patients may be taking, as they too can affect dry-eye symptoms. Oral antihistamines, for example, have been shown to induce signs and symptoms associated with ocular dryness, including increased ocular discomfort.2 Educating your patients about these important factors may help them better understand your need for a descriptive history. Ultimately, the extra time spent taking a thorough dry-eye history can be greatly rewarding and is more important than any of the tests discussed below.
Patient Questionnaires
As an addendum to patient history, patient questionnaires can yield important information used to diagnose dry eye and determine the severity of dry-eye symptoms. A well-designed questionnaire can be validated for reproducibility and should consist of relevant questions that elicit responsive answers. One example of a dry-eye questionnaire currently in use is the Ocular Surface Disease Index, a 12-question survey for dry-eye patients that’s been shown to be a reliable and valid instrument for directly assessing symptom frequency.3 The Dry Eye Quality of Life Question naire developed by Ora Inc., is a more elaborate 15-question survey, scored on a scale of 0 to 6 that focuses on patient quality of life. (Pollard S, et al.IOVS 2004;45:E-Abstract 82) Patients should be educated about the nature of dry-eye symptoms and encouraged to take note of their symptoms and record them. Most people are familiar with questionnaires and understand that they are an efficient way for doctors to gather information. They also reduce bias, as there are no verbal or visual cues to inadvertently influence the respondent.
Clinical Diagnostic Tools
Quality-of-life improvement is the targeted goal of the ophthalmologist and is best achieved when symptoms have been properly managed. However, these symptoms are typically attributable to an insufficiency or instability of the tear film or ocular surface that can be better understood by looking at the clinical signs of a patient’s dry eye through a series of basic tests.
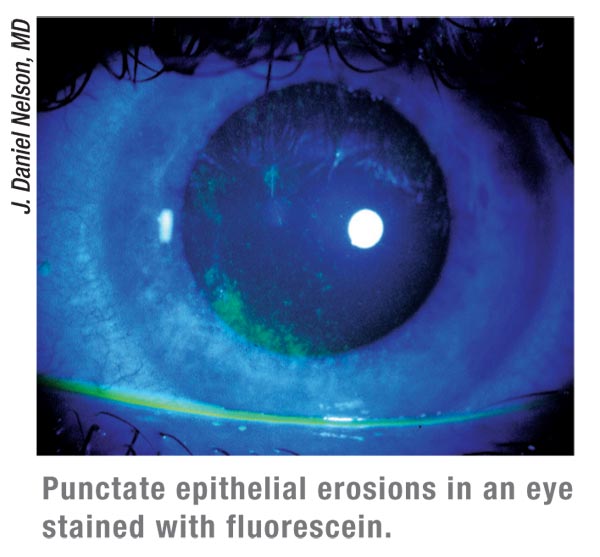
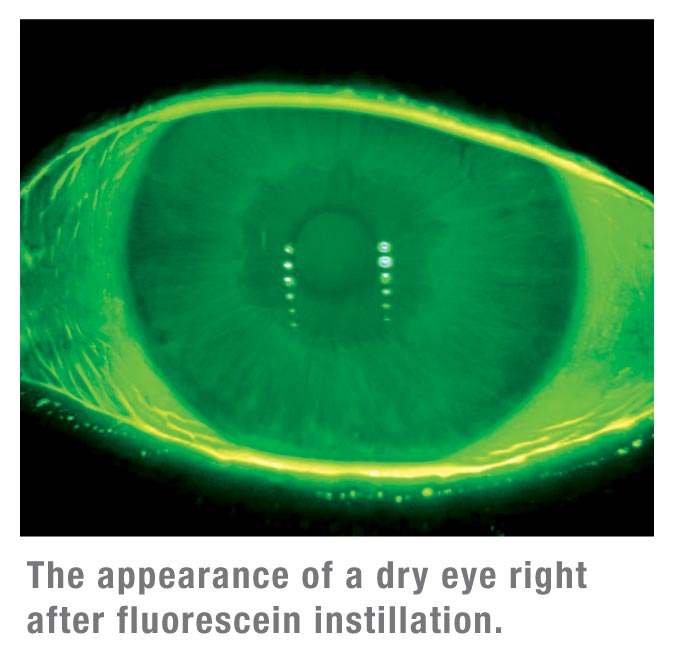
One of the most common diagnostic tests is ocular surface staining, a
critical test for determining ocular surface health. Fluorescein
staining penetrates and stains the area between surface cells, which is
optimal for determining the effects of desiccation on the corneal
surface.4 Note that large, uncontrolled amounts of fluorescein can make
it difficult to distinguish staining from an oversaturation of the
epithelium.5 A smaller, pipette-instilled drop allows for more exact
pinpointing of areas of staining and comparison of relative severity
between staining regions. Although each patient varies, the ideal time to
measure the presence of staining should be approximately three to five
minutes after drop instillation.I naccurate timing can result in ocular
surface damage that may be misrepresentative of the true health of the
ocular surface. Typically, more superficial punctate keratitis and
confluent patches of fluorescein staining are seen in patients with
more severe dry eye; however, some patients may have high levels of
fluorescein staining with minimal symptoms, which may be due to an
associated decrease in corneal sensitivity.6,7
Rose bengal and lissamine green staining have also been used as diagnostic tests, both of which stain desiccated and dying cells on the ocular surface and better target the conjunctiva. Historically, lissamine green has shown better patient acceptability than rose bengal dye, as the latter has a tendency to cause burning or stinging upon instillation. The presence of the dye itself, however, provides a rapid diagnostic test because normal patients don’t experience burning, thus demonstrating the ability of the ocular surface to deal with an external irritant. An additional difference between these two agents is that rose bengal stains both dying cells and healthy corneal cells, while lissamine green stains only the former. Ideally, a clinician would look at both fluorescein staining and lissamine staining to approximate the extent of the damage to the ocular surface.
The inferior corneal surface usually sees the most staining, as it is exposed to the elements more than other regions due to the tear meniscus decrease and incomplete blinks of the interpalpebral fissure. Research also suggests that there may be correlations between ocular discomfort and central staining, as the central cornea has five to six times as many nerve fibers as the peripheral cornea.8 One of the underlying causes of dry eye may be a decrease in goblet cell population, or a decrease in mucin cell creation from these goblet cells, which may account for increased staining in the conjunctiva. Conjunctival goblet cells are modified epithelial cells whose main function is to synthesize, store, and secret mucins.9 They are the main contributors to the mucus layer of the tear film that protects the ocular surface from exogenous agents. Mucin secretagogues, which are used to increase the secretion of mucin from goblet cells and to improve the quality of a mucin secretion, are believed to have a significant effect on lissamine staining and may be an indicator of a drug’s potential efficacy.
Ocular surface desiccation made apparent by dye staining is usually preceded by tear film instability. Tear-film instability can result in significant exposure of the cornea and ultimately, ocular surface damage and symptoms of dry eye. Tear-film breakup time measures the interval between the last complete blink and the breakup of the tear film, appearing as dark spots called micelles. This test indicates areas in which the tear film has receded from the otherwise continuous fluorescein green tear film, leaving the ocular surface exposed.5 Like ocular staining, TFBUT measurements are most accurate with well-controlled micro-quantities (1 to 5 ìl) of fluorescein.5,10-12 Instillation of a larger drop of fluorescent dye can flood the tear film and prolong TFBUT measurements. Reliable and reproducible reference values have been established in which TFBUT was determined to be greater than five seconds in normals (mean=7.1 ±1.17 seconds) and less than five seconds in dry-eye patients (mean=2.2 ±0.82 seconds).5
While the hallmark indication of dry eye has been tear-film stability, an interest in the role of blink patterns has recently surfaced. Changes in blink pattern may cause longer interblink intervals potentially resulting in exaggerated amounts of corneal exposure and resultant inflammation. It has been established that normal patients tend to have significantly longer IBIs than dry-eye patients. (White et al. IOVS 2010;51: ARVO E-Abstract952) Ultimately, those patients with an increased blink rate may experience greater discomfort than those patients who have a lower blink rate. One caveat, however is corneal sensitivity; those patients with decreased corneal sensitivity may be less likely to indicate their discomfort by an increased blink rate.
To that end, it is of interest to look at the number of years a patient has been diagnosed with dry eye, as it may play a role in both sensitivity and symptoms. (Casavant et al. IOVS 2005;52:ARVO E-Abstract 4455) Although limited attention has been paid to the importance of blink pattern, largely because blink patterns are highly variable depending on the environment and visual tasks, recent technologies have adapted to help us better understand this endpoint.
In an effort to more adequately measure the symptomatology of dry eye, a parameter known as symptomatic breakup time was established. This is the measure of the time it takes a patient to first report a sensation of ocular awareness after blinking twice then staring straight ahead. Approximately 70 percent of patients diagnosed with dry eye report ocular awareness followed by discomfort after TFBUT, suggesting that SBUT is a good estimation of breakup time.13 This test outfits patients with an awareness of their condition and an ability to gauge changes in it, and further evaluate their ocular status and assess the performance of tear substitutes. While SBUT is a useful tool for patients and clinicians, this measurement provides only a part of the necessary picture needed to comprehend the worsening of dry eye.
Tear volume and tear production are also components of tear-film stability and measurements that can be affected by dry eye. One method to determine tear volume is a Schirmer’s test, which can be done with a local anesthetic (Schirmer’s II test) or without (Schirmer’s I test). In either procedure, paper strips are placed in each eye for roughly five minutes to assess aqueous production. This test is useful in providing a clinical sign of dry eye, although it’s important to be aware of reflex tearing, which can reduce the precision of the strips. At times, the unanaesthetized placement of these strips can be somewhat uncomfortable for patients.
A clinician can conduct a more patient- friendly tear volume estimation by looking at the tear meniscus heights in the marginal menisci during a slitlamp exam. These readings can help determine if a dry-eye patient is potentially aqueous deficient.14 Additionally, fluorophotometry is an effective and reproducible method of measuring tear production. This diagnostic test determines tear turnover rate, tear volume and tear flow by measuring the decay of fluorescein in the tear film.
It’s thought that higher osmolarity readings indicate a greater likelihood that the tears being tested are less stable in composition.15 This may be a function of lipid layer insufficiency, resulting in increased tear film evaporation.
While osmolarity is the only diagnostic test that has a dedicated CPT code (83861), there appears to be much variability within its use. (Wunderlich et al. IOVS 2011;52:ARVO E-Abstract 930; Vasan et al. IOVS 2011;52:ARVO E-Abstract 927; Keech et al. IOVS 2011;52:ARVO E-Abstract 725; Langelier et al. IOVS 2011;52: ARVO E-Abstract 925) Although this biomarker requires additional work and should still be used in conjunction with a full battery of tests, it shows promise as a valuable test.
Impression cytology has also been a pivotal component of investigational studies, including assisting in the diagnosis of ocular surface disease, improving our understanding of ocular surface disease and providing biomarkers to be used as endpoints in clinical trials. In IC, superficial layers of the epithelium are removed by application of cellulose acetate filters or Biopore membranes, harvested noninvasively from the ocular surface, and subsequently analyzed by various methods.
Another option chosen by some clinicians is white-light interferometry. In it, a light is directed toward the eye and an imager captures the resulting interference pattern, which the physician uses to evaluate the tear film.17
While the exact cause of dry-eye disease is unknown, it’s likely that infl ammation of the ocular surface plays a significant role and leads to some of the observed signs and symptoms of dry eye. It’s been suggested that dry eye is a TH1-mediated disease, primarily involving the response of local cells (conjunctival epithelial cells), rather than systemic cells.18 IC in conjunction with flow cytometry, which examines particles by suspending them in a stream of fluid and passing them by an electronic detection apparatus, thus providing an objective metric, has provided further information regarding the pathophysiology of inflammation.
Finally, it’s important to evaluate the status of the meibomian glands and lid margins. Healthy meibomian glands are absolutely vital to a healthy tear film and are often responsible for a majority of dry-eye signs and symptoms.By secreting lipids that help stabilize the tear film, the meibomian glands minimize the evaporate loss of tear fluid, and any meibomian gland abnormalities may create significant ocular surface problems. There is also no harm in looking at the lacrimal gland, located in the upper outer quadrant of the orbit, which can be examined relatively easily for infiltration or obstruction.
Ophthalmologists have a variety of diagnostic approaches to evaluate a patient’s tear film instabilities and ocular surface desiccation. Nevertheless, innovation and refining of diagnostic methods and materials are crucial to tackling the disease. As dry-eye diagnostics continue to advance, the simplicity and tangibility of these standard tests make them a valuable option to both physicians and patients.
Dr. Abelson, a clinical professor of ophthalmology at Harvard Medical School, consults in ophthalmic pharmaceuticals. Mr. Ousler is director of the dry-eye department, and Ms. Lafond is a medical writer at Ora Inc., in Andover.
1. Abelson MB, Ousler GW, 3rd, Nally LA, Emory TB. Dry eye syndromes: diagnosis, clinical trials and pharmaceutical treatment—’improving clinical trials’. Adv Exp Med Biol 2002;506(Pt B):1079-1086.
2. Ousler GW, Wilcox KA, Gupta G, Abelson MB. An evaluation of the ocular drying effects of two systemic antihistamines: Loratadine and cetirizine hydrochloride. Ann Allergy Asthma Immunol 2004;93:5:460-464.
3. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. May 2000;118:5:615-621.
4. Ousler GW, Gomes PJ, Welch D, Abelson MB. Methodologies for the Study of Ocular Surface Disease. The Ocular Surface. Vol 3; 2005:143-154.
5. Abelson MB, Ousler GW, 3rd, Nally LA, Welch D, Krenzer K. Alternative reference values for TFBUT in normal and dry eye populations. Adv Exp Med Biol 2002;506(Pt B):1121-1125.
6. Xu KP, Yagi Y, Tsubota K. Decrease in corneal sensitivity and change in tear function in dry eye. Cornea 1996;15:3:235-239.
7. Bourcier T, Acosta MC, Borderie V, et al. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci 2005;46:7:2341-2345.
8. Muller LJ, Vrensen GF, Pels L, Cardozo BN, Willekens B. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci 1997;38:5:985-994.
9. Forstner G. Signal transduction, packaging and secretion of mucins. Annu Rev Physiol 1995;57:585-605.
10. Abdul-Fattah AM, Bhargava HN, Korb DR, Glonek T, Finnemore VM, Greiner JV. Quantitative in vitro comparison of fl uorescein delivery to the eye via impregnated paper strip and volumetric techniques. Optom Vis Sci 2002;79:7:435-438.
11. Korb DR, Herman JP, Finnemore VM, Exford JM, Blackie CA. An evaluation of the effi cacy of fl uorescein, rose bengal, lissamine
green, and a new dye mixture for ocular surface staining. Eye Contact Lens 2008;34:1:61-64.
12. Marquardt R SR, Christ T. Modifi cation of tear fi lm break-up time test for increased reliability. In: Holly FJ, ed. The Preocular Tear Film in Health, Disease and Contact Lens Wear. Lubbock, Texas: Dry Eye Institute, 1986:57-63.
13. Nally LA, Ousler GW, MB A. Ocular discomfort and tear film break up time in dry eye patients. Invest Ophthalmol Vis Sci 2000;41(Suppl)1436).
14. Lim KJ, Lee JH. Measurement of the tear meniscus height using 0.25% fl uorescein sodium. Korean J Ophthalmol 1991;5:1:34-36.
15. Gilbard JP. Human tear fi lm electrolyte concentrations in health and dry-eye disease. Int Ophthalmol Clin 1994;34:1:27.
16. Rolando M, Refojo MF, Kenyon KR. Increased tear evaporation in eyes with keratoconjunctivitis sicca. Arch Ophthalmol 1983;101:4:557-558.
17. Goto E, Tseng SCG. Differentiation of lipid tear defi ciency dry eye by kinetic analysis of tear interference images. Arch Ophthalmol 2003;121:173-80.
18. Singh R, Joseph A, Umapathy T, Tint NL, Dua HS. Impression cytology of the ocular surface. Br J Ophthalmol 2005;89:12:1655- 1659.
 Patient History
Patient History
Dry eye can develop in association with a number of autoimmune diseases, such as systemic lupus and rheumatoid arthritis, or be brought on and exacerbated by certain environmental and lifestyle adaptations. Careful examination and utilization of proper patient questioning and taking a detailed history can lead to a focused diagnosis of dry eye and ultimately a more rewarding treatment plan.
Patients may not be aware that they have dry eye, but may verbalize their symptoms in ways that can point you toward a diagnosis. Many patients notice changes in their symptoms at different times during the day, often noting changes in severity while driving at night or engaging in extended visual tasking activities such as watching television or using the computer. It’s also important to take note of any prescription medications your patients may be taking, as they too can affect dry-eye symptoms. Oral antihistamines, for example, have been shown to induce signs and symptoms associated with ocular dryness, including increased ocular discomfort.2 Educating your patients about these important factors may help them better understand your need for a descriptive history. Ultimately, the extra time spent taking a thorough dry-eye history can be greatly rewarding and is more important than any of the tests discussed below.
Patient Questionnaires
As an addendum to patient history, patient questionnaires can yield important information used to diagnose dry eye and determine the severity of dry-eye symptoms. A well-designed questionnaire can be validated for reproducibility and should consist of relevant questions that elicit responsive answers. One example of a dry-eye questionnaire currently in use is the Ocular Surface Disease Index, a 12-question survey for dry-eye patients that’s been shown to be a reliable and valid instrument for directly assessing symptom frequency.3 The Dry Eye Quality of Life Question naire developed by Ora Inc., is a more elaborate 15-question survey, scored on a scale of 0 to 6 that focuses on patient quality of life. (Pollard S, et al.IOVS 2004;45:E-Abstract 82) Patients should be educated about the nature of dry-eye symptoms and encouraged to take note of their symptoms and record them. Most people are familiar with questionnaires and understand that they are an efficient way for doctors to gather information. They also reduce bias, as there are no verbal or visual cues to inadvertently influence the respondent.
Clinical Diagnostic Tools
Quality-of-life improvement is the targeted goal of the ophthalmologist and is best achieved when symptoms have been properly managed. However, these symptoms are typically attributable to an insufficiency or instability of the tear film or ocular surface that can be better understood by looking at the clinical signs of a patient’s dry eye through a series of basic tests.
 |
Rose bengal and lissamine green staining have also been used as diagnostic tests, both of which stain desiccated and dying cells on the ocular surface and better target the conjunctiva. Historically, lissamine green has shown better patient acceptability than rose bengal dye, as the latter has a tendency to cause burning or stinging upon instillation. The presence of the dye itself, however, provides a rapid diagnostic test because normal patients don’t experience burning, thus demonstrating the ability of the ocular surface to deal with an external irritant. An additional difference between these two agents is that rose bengal stains both dying cells and healthy corneal cells, while lissamine green stains only the former. Ideally, a clinician would look at both fluorescein staining and lissamine staining to approximate the extent of the damage to the ocular surface.
The inferior corneal surface usually sees the most staining, as it is exposed to the elements more than other regions due to the tear meniscus decrease and incomplete blinks of the interpalpebral fissure. Research also suggests that there may be correlations between ocular discomfort and central staining, as the central cornea has five to six times as many nerve fibers as the peripheral cornea.8 One of the underlying causes of dry eye may be a decrease in goblet cell population, or a decrease in mucin cell creation from these goblet cells, which may account for increased staining in the conjunctiva. Conjunctival goblet cells are modified epithelial cells whose main function is to synthesize, store, and secret mucins.9 They are the main contributors to the mucus layer of the tear film that protects the ocular surface from exogenous agents. Mucin secretagogues, which are used to increase the secretion of mucin from goblet cells and to improve the quality of a mucin secretion, are believed to have a significant effect on lissamine staining and may be an indicator of a drug’s potential efficacy.
Ocular surface desiccation made apparent by dye staining is usually preceded by tear film instability. Tear-film instability can result in significant exposure of the cornea and ultimately, ocular surface damage and symptoms of dry eye. Tear-film breakup time measures the interval between the last complete blink and the breakup of the tear film, appearing as dark spots called micelles. This test indicates areas in which the tear film has receded from the otherwise continuous fluorescein green tear film, leaving the ocular surface exposed.5 Like ocular staining, TFBUT measurements are most accurate with well-controlled micro-quantities (1 to 5 ìl) of fluorescein.5,10-12 Instillation of a larger drop of fluorescent dye can flood the tear film and prolong TFBUT measurements. Reliable and reproducible reference values have been established in which TFBUT was determined to be greater than five seconds in normals (mean=7.1 ±1.17 seconds) and less than five seconds in dry-eye patients (mean=2.2 ±0.82 seconds).5
While the hallmark indication of dry eye has been tear-film stability, an interest in the role of blink patterns has recently surfaced. Changes in blink pattern may cause longer interblink intervals potentially resulting in exaggerated amounts of corneal exposure and resultant inflammation. It has been established that normal patients tend to have significantly longer IBIs than dry-eye patients. (White et al. IOVS 2010;51: ARVO E-Abstract952) Ultimately, those patients with an increased blink rate may experience greater discomfort than those patients who have a lower blink rate. One caveat, however is corneal sensitivity; those patients with decreased corneal sensitivity may be less likely to indicate their discomfort by an increased blink rate.
To that end, it is of interest to look at the number of years a patient has been diagnosed with dry eye, as it may play a role in both sensitivity and symptoms. (Casavant et al. IOVS 2005;52:ARVO E-Abstract 4455) Although limited attention has been paid to the importance of blink pattern, largely because blink patterns are highly variable depending on the environment and visual tasks, recent technologies have adapted to help us better understand this endpoint.
In an effort to more adequately measure the symptomatology of dry eye, a parameter known as symptomatic breakup time was established. This is the measure of the time it takes a patient to first report a sensation of ocular awareness after blinking twice then staring straight ahead. Approximately 70 percent of patients diagnosed with dry eye report ocular awareness followed by discomfort after TFBUT, suggesting that SBUT is a good estimation of breakup time.13 This test outfits patients with an awareness of their condition and an ability to gauge changes in it, and further evaluate their ocular status and assess the performance of tear substitutes. While SBUT is a useful tool for patients and clinicians, this measurement provides only a part of the necessary picture needed to comprehend the worsening of dry eye.
Tear volume and tear production are also components of tear-film stability and measurements that can be affected by dry eye. One method to determine tear volume is a Schirmer’s test, which can be done with a local anesthetic (Schirmer’s II test) or without (Schirmer’s I test). In either procedure, paper strips are placed in each eye for roughly five minutes to assess aqueous production. This test is useful in providing a clinical sign of dry eye, although it’s important to be aware of reflex tearing, which can reduce the precision of the strips. At times, the unanaesthetized placement of these strips can be somewhat uncomfortable for patients.
A clinician can conduct a more patient- friendly tear volume estimation by looking at the tear meniscus heights in the marginal menisci during a slitlamp exam. These readings can help determine if a dry-eye patient is potentially aqueous deficient.14 Additionally, fluorophotometry is an effective and reproducible method of measuring tear production. This diagnostic test determines tear turnover rate, tear volume and tear flow by measuring the decay of fluorescein in the tear film.
It’s thought that higher osmolarity readings indicate a greater likelihood that the tears being tested are less stable in composition.15 This may be a function of lipid layer insufficiency, resulting in increased tear film evaporation.
While osmolarity is the only diagnostic test that has a dedicated CPT code (83861), there appears to be much variability within its use. (Wunderlich et al. IOVS 2011;52:ARVO E-Abstract 930; Vasan et al. IOVS 2011;52:ARVO E-Abstract 927; Keech et al. IOVS 2011;52:ARVO E-Abstract 725; Langelier et al. IOVS 2011;52: ARVO E-Abstract 925) Although this biomarker requires additional work and should still be used in conjunction with a full battery of tests, it shows promise as a valuable test.
Impression cytology has also been a pivotal component of investigational studies, including assisting in the diagnosis of ocular surface disease, improving our understanding of ocular surface disease and providing biomarkers to be used as endpoints in clinical trials. In IC, superficial layers of the epithelium are removed by application of cellulose acetate filters or Biopore membranes, harvested noninvasively from the ocular surface, and subsequently analyzed by various methods.
Another option chosen by some clinicians is white-light interferometry. In it, a light is directed toward the eye and an imager captures the resulting interference pattern, which the physician uses to evaluate the tear film.17
While the exact cause of dry-eye disease is unknown, it’s likely that infl ammation of the ocular surface plays a significant role and leads to some of the observed signs and symptoms of dry eye. It’s been suggested that dry eye is a TH1-mediated disease, primarily involving the response of local cells (conjunctival epithelial cells), rather than systemic cells.18 IC in conjunction with flow cytometry, which examines particles by suspending them in a stream of fluid and passing them by an electronic detection apparatus, thus providing an objective metric, has provided further information regarding the pathophysiology of inflammation.
Finally, it’s important to evaluate the status of the meibomian glands and lid margins. Healthy meibomian glands are absolutely vital to a healthy tear film and are often responsible for a majority of dry-eye signs and symptoms.By secreting lipids that help stabilize the tear film, the meibomian glands minimize the evaporate loss of tear fluid, and any meibomian gland abnormalities may create significant ocular surface problems. There is also no harm in looking at the lacrimal gland, located in the upper outer quadrant of the orbit, which can be examined relatively easily for infiltration or obstruction.
Ophthalmologists have a variety of diagnostic approaches to evaluate a patient’s tear film instabilities and ocular surface desiccation. Nevertheless, innovation and refining of diagnostic methods and materials are crucial to tackling the disease. As dry-eye diagnostics continue to advance, the simplicity and tangibility of these standard tests make them a valuable option to both physicians and patients.
Dr. Abelson, a clinical professor of ophthalmology at Harvard Medical School, consults in ophthalmic pharmaceuticals. Mr. Ousler is director of the dry-eye department, and Ms. Lafond is a medical writer at Ora Inc., in Andover.
1. Abelson MB, Ousler GW, 3rd, Nally LA, Emory TB. Dry eye syndromes: diagnosis, clinical trials and pharmaceutical treatment—’improving clinical trials’. Adv Exp Med Biol 2002;506(Pt B):1079-1086.
2. Ousler GW, Wilcox KA, Gupta G, Abelson MB. An evaluation of the ocular drying effects of two systemic antihistamines: Loratadine and cetirizine hydrochloride. Ann Allergy Asthma Immunol 2004;93:5:460-464.
3. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. May 2000;118:5:615-621.
4. Ousler GW, Gomes PJ, Welch D, Abelson MB. Methodologies for the Study of Ocular Surface Disease. The Ocular Surface. Vol 3; 2005:143-154.
5. Abelson MB, Ousler GW, 3rd, Nally LA, Welch D, Krenzer K. Alternative reference values for TFBUT in normal and dry eye populations. Adv Exp Med Biol 2002;506(Pt B):1121-1125.
6. Xu KP, Yagi Y, Tsubota K. Decrease in corneal sensitivity and change in tear function in dry eye. Cornea 1996;15:3:235-239.
7. Bourcier T, Acosta MC, Borderie V, et al. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci 2005;46:7:2341-2345.
8. Muller LJ, Vrensen GF, Pels L, Cardozo BN, Willekens B. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci 1997;38:5:985-994.
9. Forstner G. Signal transduction, packaging and secretion of mucins. Annu Rev Physiol 1995;57:585-605.
10. Abdul-Fattah AM, Bhargava HN, Korb DR, Glonek T, Finnemore VM, Greiner JV. Quantitative in vitro comparison of fl uorescein delivery to the eye via impregnated paper strip and volumetric techniques. Optom Vis Sci 2002;79:7:435-438.
11. Korb DR, Herman JP, Finnemore VM, Exford JM, Blackie CA. An evaluation of the effi cacy of fl uorescein, rose bengal, lissamine
green, and a new dye mixture for ocular surface staining. Eye Contact Lens 2008;34:1:61-64.
12. Marquardt R SR, Christ T. Modifi cation of tear fi lm break-up time test for increased reliability. In: Holly FJ, ed. The Preocular Tear Film in Health, Disease and Contact Lens Wear. Lubbock, Texas: Dry Eye Institute, 1986:57-63.
13. Nally LA, Ousler GW, MB A. Ocular discomfort and tear film break up time in dry eye patients. Invest Ophthalmol Vis Sci 2000;41(Suppl)1436).
14. Lim KJ, Lee JH. Measurement of the tear meniscus height using 0.25% fl uorescein sodium. Korean J Ophthalmol 1991;5:1:34-36.
15. Gilbard JP. Human tear fi lm electrolyte concentrations in health and dry-eye disease. Int Ophthalmol Clin 1994;34:1:27.
16. Rolando M, Refojo MF, Kenyon KR. Increased tear evaporation in eyes with keratoconjunctivitis sicca. Arch Ophthalmol 1983;101:4:557-558.
17. Goto E, Tseng SCG. Differentiation of lipid tear defi ciency dry eye by kinetic analysis of tear interference images. Arch Ophthalmol 2003;121:173-80.
18. Singh R, Joseph A, Umapathy T, Tint NL, Dua HS. Impression cytology of the ocular surface. Br J Ophthalmol 2005;89:12:1655- 1659.