One of the most difficult dilemmas in the management of eyes with complex glaucoma that have already had a tube shunt implant is what to do when the tube shunt fails. Assuming that we’ve exhausted medical options, we have to decide what the next step should be. Should we replace the tube shunt? Should we implant a second one? Should we perform an additional surgical or laser procedure?
These questions are not always easy to answer, and guidance in the literature is sparse. Here, to help you decide how to proceed when faced with one of these patients, I’d like to summarize the pros and cons of many of the options we have available at this point in
Defining “Failure”
There’s no question that tube shunts often fail. In the literature, the reported rates of failure of a primary tube shunt vary from about 9 to 50 percent, depending on a number of factors, including which device was used, the definition of failure used in the study, the design of the study and how long the patients were followed after the procedure. Most of the retrospective studies in the literature show higher rates of tube shunt failure between the third and sixth years, with upwards of 30 to 50 percent of tubes failing during that period.
One of the reasons for the widely varying reports of success or failure in the clinical data is that there’s no agreed-upon definition of tube-shunt “failure.” Each research protocol or study chooses a specific target to help decide whether the treatments being tested are effective. That usually means picking a target pressure to
 | |
| Click to enlarge. |
Not surprisingly, any marker you choose also has the potential to create a somewhat misleading conclusion. For example, if you set your “failure pressure” at 18 mmHg and a given patient ends up at 19 mmHg, that patient is considered to have failed, even if her glaucoma is under control and not progressing. (In other words, the tube shunt didn’t really fail.) On the other hand, you could have patients with a pressure of 15 mmHg who are still progressing. Those patients would be counted as “successes” in that particular study, even though the patients’ condition would continue to worsen.
Of course, a good randomized, prospective study of the different options would help provide a lot of answers, but up until now, nobody’s really looked at this question prospectively. There was a trial sponsored by the Centers for Disease Control and Prevention that was designed to look at how different tube-shunt options compare, but the primary endpoint was a comparison of success between the interventions, not which worked best as a second surgical treatment. The American Glaucoma Society is sponsoring a trial, currently in the recruitment phase, to compare a second Baerveldt shunt to diode
be a few years before that produces meaningful results.
For the purposes of this article, I’m defining primary tube-shunt failure as the inability to prevent
Managing Tube Number One
When we find that a patient with a tube shunt still has insufficiently controlled pressure, our first mission is to figure out why. To do that, we need to know whether the first tube shunt is still functioning. Is there fluid flowing into the reservoir or not? In some cases, the tube may still be functioning, but insufficient for getting the pressure down to your target range; in other cases, the shunt may have stopped working.
 |
There are a couple of ways to determine whether the shunt is still working. One is to use an ultrasound B-scan to see if there’s a hypoechoic shadow or border around the plate and the tube. A second option is to place a small-gauge needle on a syringe, prep the patient in a sterile manner, and then try to aspirate fluid from the reservoir over the plates; if you can’t get anything out, then you know the tube is occluded. You can also push on the eye a little bit and see if the pressure drops and the reservoir comes up. (With the last two options, of course, you need to watch the anterior chamber carefully, because if the tube is actually working, you could theoretically collapse the anterior chamber by pulling or pushing out too much fluid.) The bottom line is, if there’s fluid around the plate, the tube is functioning to some degree; it’s just not working well enough to help that patient. In that situation, you need to add another pressure-lowering option. (More on that below.)
If you’ve determined that the tube isn’t working at all, you can either revise the tube to try to get it working again or replace it. One option is to try to flush out the tube in the OR using a 30-cc syringe. To do this, you pull the tube out of the eye and use a cannula loaded onto a large 30-cc syringe to forcefully irrigate the tube, in order to either expand or fracture the capsule of fibrous tissue that forms around the plate and tube. Another option is to try resecting the capsule from around the tube so that it’s not restricting the volume the tube can hold.
This type of approach has been reported to have a success rate as high as 50 percent, but again, this depends on what the surgeons used for their definitions of success and how long they followed the patients. One published study compared the outcomes of resecting the capsule to putting in a second tube shunt.1 The second-tube option did a better job of controlling
Another option is to replace the first tube with a different type of tube. There’s almost no published data regarding this approach, even though many surgeons report doing this. However, a very small series was recently published in which the surgeons removed failed Ahmeds and replaced them with Baerveldts. Two cases out of nine failed after five to eight years.2 Given the small size of the series, of course, this isn’t definitive information.
Moving to Step Two
If the first tube is still working but it’s not adequate to control
• Implanting
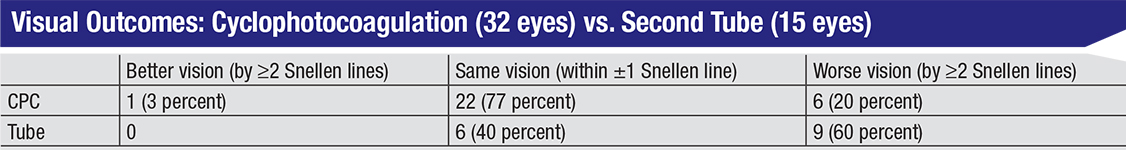 |
A second implant can definitely work, but it increases the risk of corneal edema and failure. In a retrospective study conducted by one group,3 there also seemed to be a risk of exposure of the first implant. We don’t know if that was because of tissue manipulation, desiccation or some other factor. A second tube shunt certainly is not a panacea; additional interventions that were required after a second tube shunt have been reported because of tube erosion, corneal failure, endophthalmitis
Another key question is this: If you opt to implant a second tube, should you implant the same device or a different tube shunt? If an Ahmed isn’t working, for example, some surgeons will switch to a Baerveldt or Molteno to see if they’ll get a better result. Unfortunately, published data is scarce on the effectiveness of implanting the same device or trying one of the others; the surgeon has to use his or her best judgment regarding the cause of the first tube’s failure and whether that suggests that a different device might work better.
When deciding whether to implant a second tube, another factor worth considering is the impact of having a second piece of hardware on the eye, in terms of vision. For example, diplopia can be an issue in this situation. Of course, many of these patients have
• Performing a trabeculectomy. Most surgeons wouldn’t do a filtering procedure like a trabeculectomy with a tube already in place. I haven’t seen any published data on the success of such an approach, but the patient isn’t likely to do well because the conjunctiva is already scarred. (Whether there’s a role for a device like the Xen or InnFocus, which are bleb-creators, remains to be seen.)
• Performing a
 |
It’s true that patients in some of the early studies required additional interventions, but many of those were actually extra CPC treatments. The reason for this was that when this option was first being investigated, surgeons were very conservative in the initial laser treatment, so they often undertreated. Up to that point, CPC had usually been reserved for eyes that were blind, or almost blind, because CPC was thought to create so much inflammation that it could put the eye at significant risk. In fact, we’ve learned that it’s effective to start with a very conservative CPC and then follow up with additional treatments, as needed.
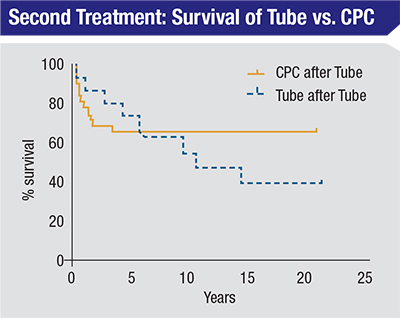
The group at the McGovern Medical School in Houston also retrospectively compared complications from a second tube shunt to complications from CPC; that data also favored CPC, in terms of avoiding serious complications, but the CPC group did require more interventions. The second-tube-shunt group had more serious complications, including three cases of endophthalmitis and more persistent corneal edema. This was also observed in the second-tube groups in some of the retrospective studies mentioned earlier.
There’s
The Big Picture
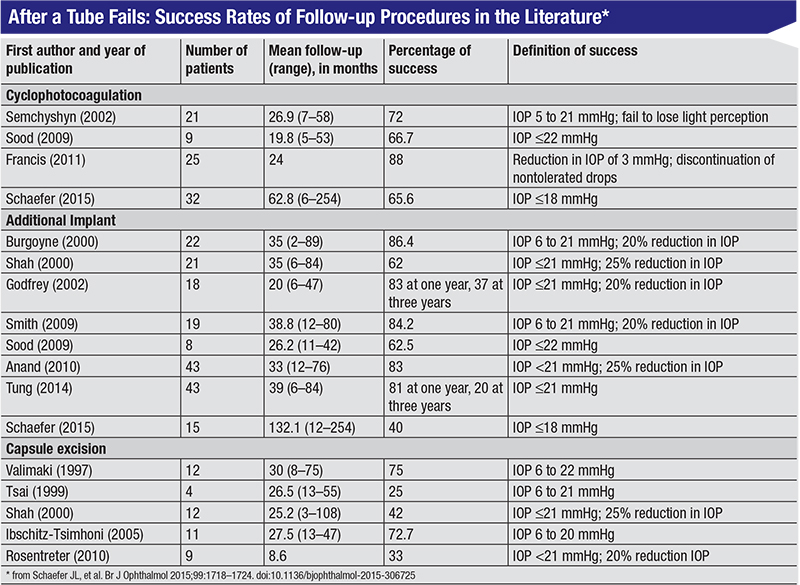
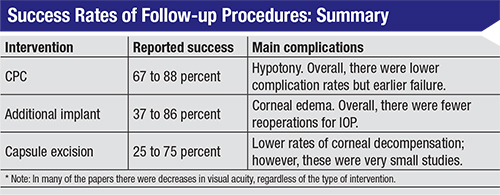
The table on page 99, assembled by Jamie Schaefer, MD, et al, summarizes success rates for different follow-up procedures, reported in multiple studies. For CPC that’s used after a primary tube shunt fails, the reported success rates ranged from 67 to 88 percent; the main complication was hypotony. Overall the CPC group had
We don’t yet have much information on the success rates of some of the newer procedures as
| When deciding how to proceed, you need to weigh the risks and benefits based on the patient sitting in front of you, looking for the least-offensive option to help that already sick eye. |
The new
Helping the Patient
What about my own experience? Initially, I was doing a lot of second tubes in these patients, but when the retrospective data started coming out, I began opting more often for CPC. It’s technically easier to perform, it’s quicker, and the patient has a quicker recovery. Of course, we don’t really know if the laser is the better option—yet.
These are difficult patients. Buying the patient as much time as possible is all you can really do, and every patient is different. There’s no standard approach. Whenever we do any kind of invasive glaucoma surgery, we’ve already determined that the patient will go blind from glaucoma at some point, and a small risk associated with an intervention is almost always less than the risk of the person going permanently blind. (That being said, some risks are more serious than others. Glaucoma can blind a person over a period of years, but endophthalmitis can do it in a week.) When deciding how to proceed, you need to weigh the risks and benefits based on the patient sitting in front of you, looking for the least-offensive option to help that already sick eye. REVIEW
Dr. Blieden is an assistant professor at the Cullen Eye Institute, Baylor College of Medicine in Houston. She has no relevant financial
1. Shah AA, WuDunn D, Cantor LB. Shunt revision versus additional tube shunt implantation after failed tube shunt surgery in refractory glaucoma. Am J Ophthalmol 2000;129:4:455-60.
2. Zuo W, Lesk MR. Surgical outcome of replacing a failed Ahmed glaucoma valve by a Baerveldt glaucoma implant in the same quadrant in refractory glaucoma. J Glaucoma 2018;27:5:421-428.
3. Wang MY, Patel K, Blieden LS, et al. Comparison of Efficacy and Complications of Cyclophotocoagulation and second glaucoma drainage device after initial glaucoma drainage device failure. J Glaucoma 2017;26:11:1010-1018.
4. Francis BA, Kawji AS, Vo NT, Dustin L, Chopra V. Endoscopic
5. Schaefer JL, Levine MA, Martorana G, et al. Failed glaucoma drainage implant: long-term outcomes of a second glaucoma drainage device versus
6. Mosaed S, Chak G, Haider A, et al. Results of Trabectome surgery following failed glaucoma tube shunt implantation: Cohort study. Medicine (Baltimore) 2015;94:30:e1045.