Though diffuse unilateral subacute neuroretinitis is a rare condition, it has a long history in the medical literature. Since 1952, multiple cases of unilateral vision loss with subretinal roundworm have been reported. Diagnosing DUSN can be a challenge, since patients can be asymptomatic in the early stages and the worm is visible in less than half of all cases. Here, we’ll break down the etiology of DUSN, how it causes vision loss and the ways you can confidently diagnose and treat it if one of your patients presents with it.
Etiology
In a 1978 paper, J. Donald M. Gass, MD, and Ronald Scelfo, MD, proposed the term DUSN, instead of “unilateral wipe-out syndrome” for patients with evidence of severe central and peripheral vision loss, associated with retina and optic nerve inflammation.1
The primary cause of DUSN remained unclear until motile subretinal nematodes were found in two patients in 1978.2 In 1983, Dr. Gass and Robert Braunstein, MD, presented further evidence proving that different species of nematodes play roles in the pathogenesis of DUSN.3 Research has since been done to identify the nematodes involved. In one study, Eduardo Cunha De Souza, MD, and Yasuyuki Nakashima, MD, recovered the nematode through aspiration of subretinal material. However, the material subsequently deteriorated, making a confirmatory histopathologic investigation impossible.4 Newer techniques such as polymerase chain reaction amplification and sequencing analysis can be used to identify the nematode if the worm can be recovered from the eye.5
Due to the lack of specific serologic testing or stool examination, diagnosis of the etiologic agent of DUSN is mostly based on a combination of clinical, morphological and epidemiological information. Most of the reported cases are infected by two different sizes of worms: small worms (400 to 700 µm), including Toxocara Canis and Ancylostoma Caninum; and larger worms (1,000 to 2,000 µm), including Baylisascaris Procyonis. Larger nematodes (4,500 to 17,000 µm) have rarely been reported in the literature.6,7 Smaller worms are more commonly seen in the southeastern United States, Caribbean Islands, Latin America and South America. Larger nematodes are endemic in the northeastern and midwestern United States.8 However, nematode species are not restricted to endemic areas and occasional cases have been reported worldwide.
Pathogenesis
DUSN mostly affects healthy children and young adults with no past ocular problems. It usually involves one eye, though reports of bilateral cases are found in the literature.9 Infection is usually through the fecal-oral pathway. After entering the body, larvae migrate to the subretinal space, where they may spend months or years without changing size or shape. The worm’s migration through the retina, coupled with released toxins and subsequent inflammatory reaction, is thought to play role in the pathogenesis of DUSN and cause damage to the inner and outer retina.8
Clinical Manifestation
In the early stage, most patients are asymptomatic with or without central or paracentral scotoma. Acute unilateral visual loss may occur due to vitritis and optic disc edema. Recurrent multifocal regions of white-yellowish evanescent lesions in the outer retina and choroid are common at this stage and are attributed to reactions to the presence of nematode in the subretinal space. These lesions are typically concentrated in one segment but can migrate to other areas based on the worm’s location and movement. Lesions usually disappear within a few weeks and can leave persistent scarring and retinal pigment mottling.10 Longer worms are more likely to leave tracts of RPE clumping, whereas shorter worms cause atrophic chorioretinal scars.11 Less common abnormalities in the acute phase include ocular discomfort, congestion, iridocyclitis, subretinal hemorrhage, serous exudation, macular cysts, perivenous exudation, local retinal detachments and choroidal neovascularization.12,13
In the late stage of DUSN, patients may present with degenerative changes in the retina, particularly in the retinal pigment epithelium. As the disease progresses, retinal vessels become narrow and progressive ganglion cell loss occurs, leading to optic atrophy and profound vision loss. Additional signs of DUSN include an afferent pupillary defect, mild to moderate form of vitritis, increased internal limiting membrane reflex (Oréfice’s sign), multiple choroidal lesions and subretinal tunnels (Garcia’s sign).8,14,15
Diagnosis
A definitive diagnosis of DUSN is, of course, seeing the worm in the eye. However, patients with clinical and diagnostic features of the disease without visualization of the worm are also classified as presumed DUSN. The worm is visible during eye examinations in only 25 to 40 percent of cases as a motile, white, glistening nematode that tapers at both ends. The examination light may cause the worm to coil and uncoil slowly or, less frequently, it may slither snake-like in the subretinal space, making it more visible. The most common location for finding the worm is the posterior pole, near the edge of the gray-white lesions. There’s also a higher chance of identifying the nematode in younger individuals.8 In cases with unilateral chorioretinitis, specialists need to maintain a high level of suspicion and perform meticulous serial exams to detect the worm in this rare condition. Scanning laser ophthalmoscopy with high-contrast images and ultra-widefield photos could also be helpful in identifying the worms.16 Moreover, a recent study by the U.K.’s Simrat K. Sodhi, MD, and co-workers suggested that using a blue-light laser would cause the worm to move around, helping the clinician detect its location.17
The following tests are helpful when faced with a possible DUSN patient:
- Fluorescein angiography. Focal white-gray lesions demonstrate an early hypofluorescence followed by late staining. This might be accompanied by dye leakage around the optic disc or in a perivenous pattern. As the disease progresses and RPE loss becomes worse, fluorescein angiography manifests increased background choroidal fluorescence and a window defect.11
- Indocyanine green angiography. Early-stage DUSN is characterized by hypofluorescent dark spots, some of which are persistent, and others that become isofluorescent in the advanced stage. Persistent dots are most likely caused by full-thickness choroidal infiltration preventing ICG diffusion, whereas isofluorescent areas are partial-thickness lesions that gradually get encircled by the dye of surrounding tissues.18
- Electrophysiologic testing. Autoimmune, inflammatory, and/or toxic insult to the retinal bipolar cells can cause a mild to moderate decrease in rod and cone function, with the b-wave being more affected than the a-wave. Patients with DUSN exhibit a negative electroretinogram, which is evidenced by a flat, below-normal response of the b-wave and a decline in the b-wave to a-wave ratio.18
- Visual field studies. Various patterns of visual field loss have been reported, which correspond to the areas of chorioretinal lesions.
- OCT. All retinal layers can be impacted in DUSN. Neuroretinal atrophy, loss of the inner retinal layers, and hyperreflectivity of the regions affected by the worms have been reported.11 Some studies have demonstrated progressive atrophy in retinal nerve fiber layers associated with poor visual outcomes in the affected eye.19 En face OCT scans may provide clear visualization of the worm and highlight hyporeflective, well-delineated sections, representing vitreous lacunae created by the worm movement.17 OCT angiography can also be used in the diagnosis of DUSN by detecting the worm’s movement. Because nematodes lack blood vessels, however, they can’t be identified while they’re motionless.20
- Blood and stool test. Mild eosinophilia can be seen in patients. However, no specific change in the serologic, peripheral blood smear and stool examinations is seen and these tests have little diagnostic value in DUSN.
Case Report
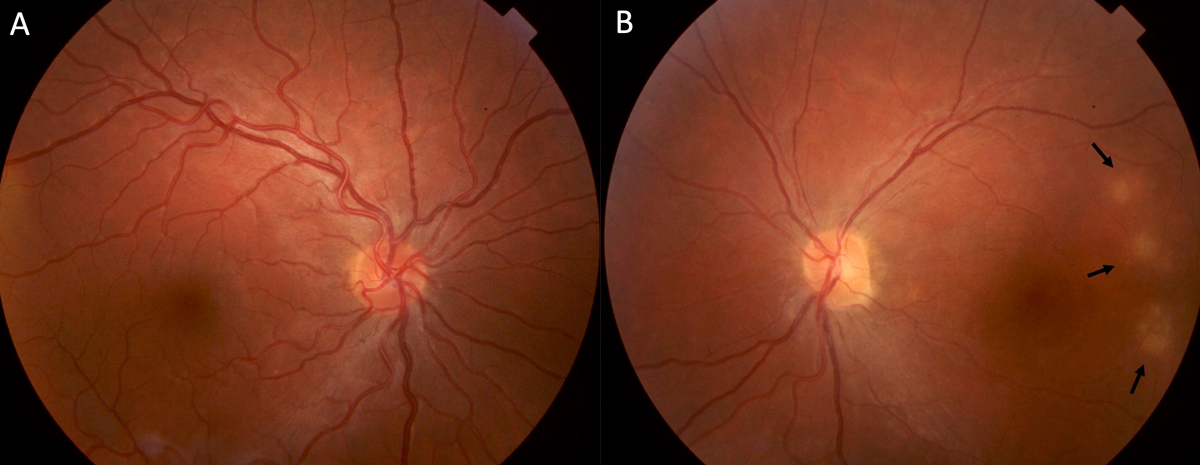
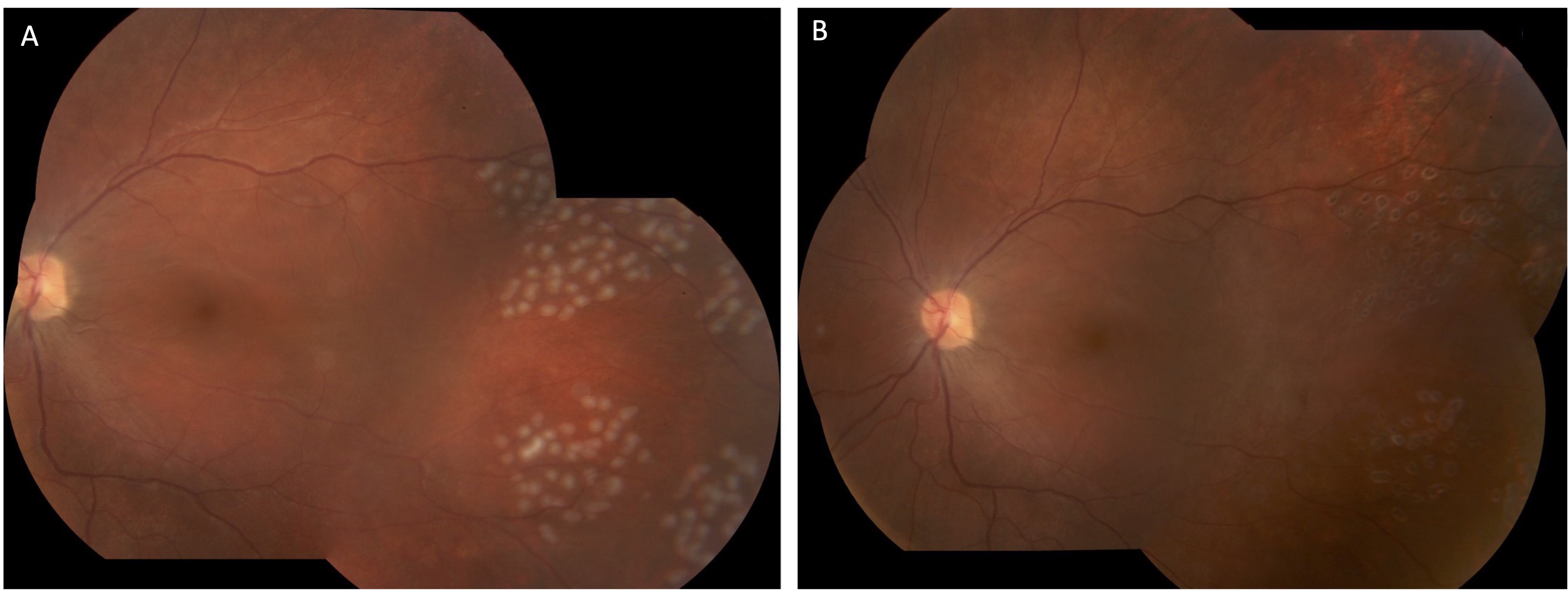
A 23-year-old man from Uruguay presented to the clinic with progressively worsening white cloud in vision in the left eye (OS) starting two years prior. Patient denied eye pain or redness. On examination, the best-corrected visual acuity was 20/20 in the right eye and counting fingers in the left. Intraocular pressure was normal. There was a left relative afferent pupillary defect. Retinal exam of the right eye was unremarkable and in the left eye multiple hypopigmented chorioretinal lesions were noted temporal to the macula (Figure 1).
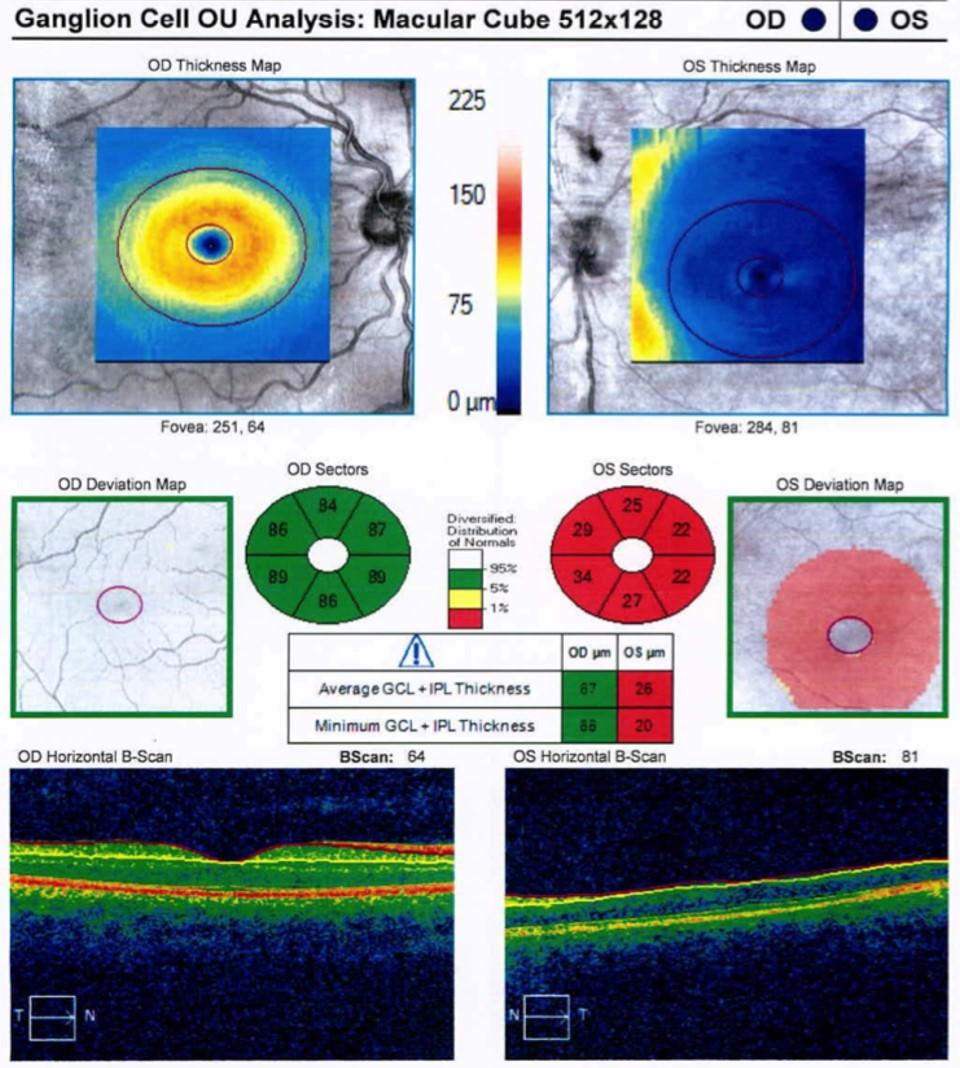
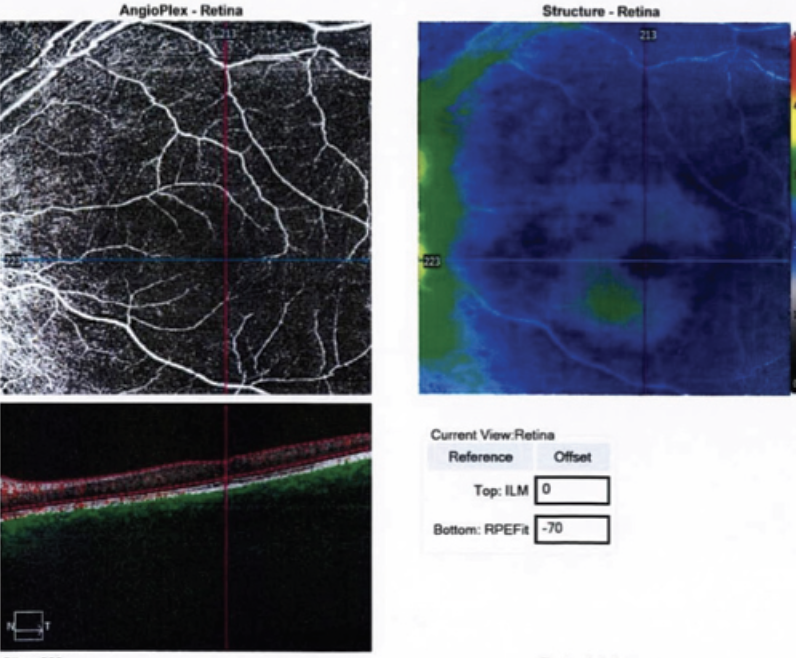
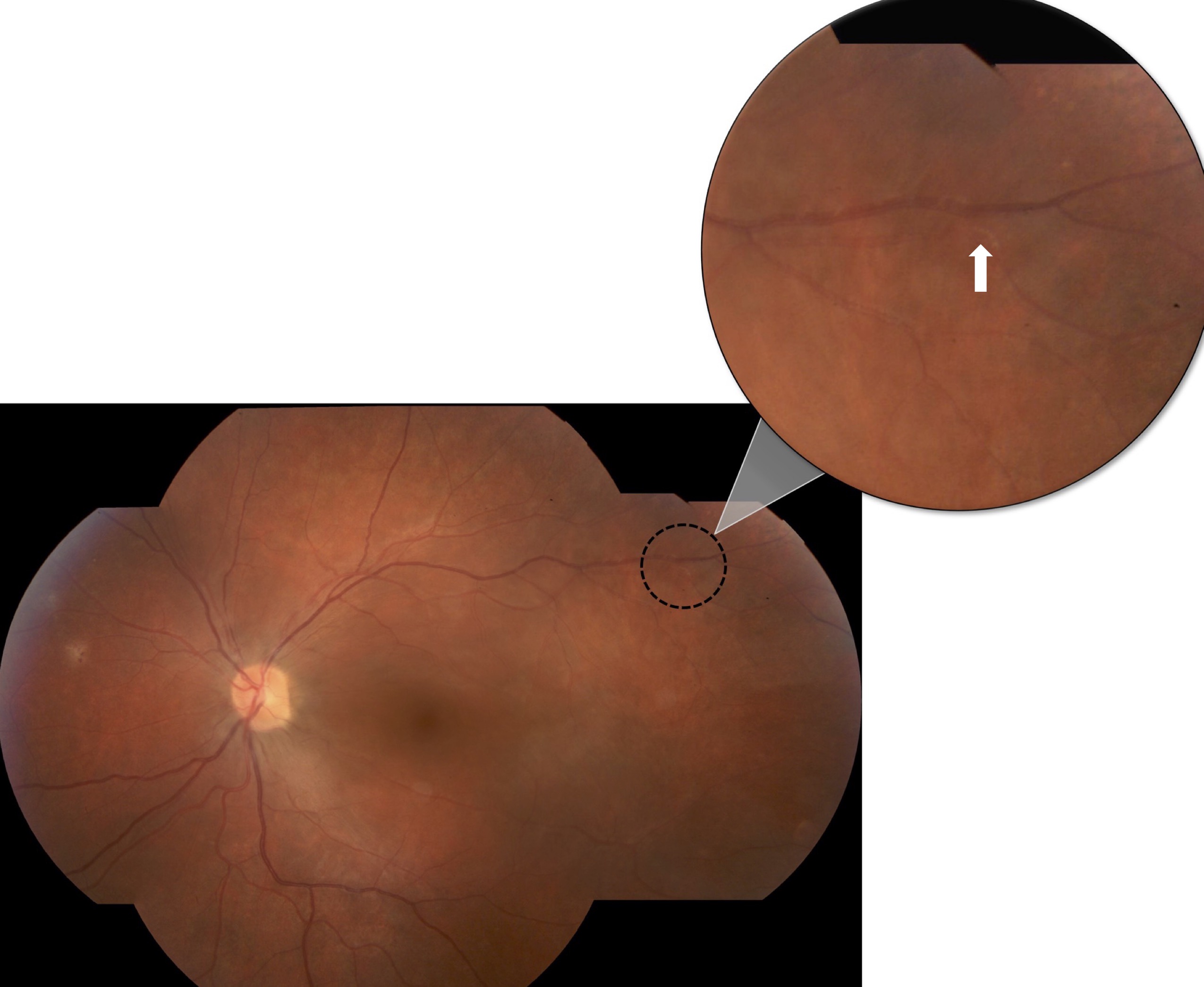
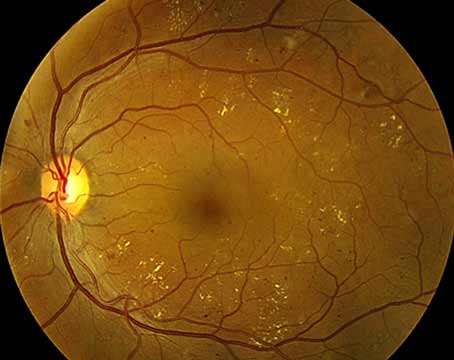
Further investigations revealed significant thinning of the retina OS on optical coherence tomography and mildly decreased flow on OCT angiography (Figures 2 and 3). Systemic work-up was performed which revealed a negative venereal disease research laboratory (VDRL) test and interferon-gamma release assay for tuberculosis. With the working diagnosis of diffuse unilateral subacute neuroretinitis, the patient received 400 mg/d of oral albendazoles. Fundus examination performed a month later clearly localized the worm, which was then barricaded with laser photocoagulation (Figures 4 and 5).
|
Treatment
When the worm is localized, laser photocoagulation can be used to kill, barricade or stop it from moving and causing further damage. To avoid a central scotoma and visual field defect, laser treatment should be administered distant from the fovea. To achieve this, a very low laser intensity or light application can cause the worm to flee to the periphery and be eliminated with minimal collateral harm.12 In cases with no detectable worm, patients are best treated by a combination of oral anthelmintic drugs, corticosteroids and scattered laser photocoagulation. The oral anthelmintic albendazole crosses the blood-retinal barrier more efficiently than thiabendazole and demonstrated remarkable results in DUSN patients. In one study, after receiving albendazole for one month, patient’s visual acuity improved from counting fingers to 20/30.21 Corticosteroids may also be used to reduce inflammation, particularly following the nematode death. The dosage and duration of treatment with anthelmintic drugs remain unclear. Most studies have suggested 400 mg/d oral albendazole for 30 days. However, short-term albendazole combined with steroid therapy has also been proposed.22
Prognosis
If the patient is diagnosed and treated at the early stages, visual acuity might be preserved. However, diagnosis is delayed in most cases, which can result in permanent visual field loss. It’s important for ophthalmologists to keep DUSN in mind as a differential diagnosis of unilateral (or bilateral) vision loss and chorioretinitis in young patients from endemic areas. Serial examinations along with other diagnostic methods are helpful for early diagnosis and the prevention of poor outcomes.
Corresponding author:
Parisa Emami-Naeini, MD, MPH
University of California, Davis Eye Center
Tschannen Eye Institute
4860 Y St
Sacramento, CA 95817
Phone: (916) 734-6602
Email: Parisaemami@gmail.com
Fateme Montazeri, MD, MPH is postdoctoral research fellow at the University of California Davis.
Emilio Dodds, MD is an ophthalmologist and uveitis specialist in Buenos Aires, Argentina.
Parisa Emami-Naeini, MD, MPH is a vitreoretinal surgeon and uveitis specialist and an assistant professor of ophthalmology and the University of California Davis. She is also the director of the uveitis service.
1. Gass JD, Scelfo R. Diffuse unilateral subacute neuroretinitis. J R Soc Med 1978;71:95–111.
2. Gass JDM, Gilbert WR, Guerry RK, Scelfo R. Diffuse unilateral subacute neuroretinitis. Ophthalmology 1978;85:521–545.
3. Gass JDM, Braunstein RA. Further observations concerning the diffuse unilateral subacute neuroretinitis syndrome. Arch Ophthalmol 1983;101:1689–1697.
4. de Souza EC, Nakashima Y. Diffuse unilateral subacute neuroretinitis. Report of transvitreal surgical removal of a subretinal nematode. Ophthalmology 1995;102:1183–1186.
5. Poppert S. et al. Diffuse unilateral subacute neuroretinitis caused by ancylostoma hookworm. Emerg Infect Dis 2017;23;343–344.
6. Kunavisarut P, Patikulsila D, Somboon P, Pathanapitoon K, Rothova A. Subretinal thelazia-induced diffuse unilateral subacute neuroretinitis. JAMA Ophthalmol 2014;132:896–898.
7. Ramachandran O, Mallidi R, Sen S, Kannan NB. Multimodal imaging-guided diagnosis, management and follow-up of a case of diffuse unilateral subacute neuroretinitis. BMJ Case Rep 2020;13:1–3.
8. Yu, H. Inflamatory and Infectious Ocular Disorders. In: Retina Atlas. New York: Springer, 2019.
9. de Souza EC, Abujamra S, Nakashima Y, Gass JD. Diffuse bilateral subacute neuroretinitis: First patient with documented nematodes in both eyes. Arch Ophthalmol 1999;117:1349–1351.
10. Patel S, Agarwal A. DUSN. In: Gupta V., Nguyen QD., LeHoang P, Agarwal A, eds. The Uveitis Atlas. Delhi, India: Springer, 2020. 413–420.
11. Mazzeo TJ, dos Santos M, Curi AL. Diffuse unilateral subacute neuroretinitis: Review article. J Ophthalmic Inflamm Infect 2019;9:23.
12. Balasopoulou A, et al. Symposium recent advances and challenges in the management of retinoblastoma globe ‑ saving treatments. BMC Ophthalmol 2017;17:1.
13. Deb AK, Jawahar SB, Priyanka R, et al. Multifocal chorioretinitis with serous macular detachment in diffuse unilateral subacute neuroretinitis (DUSN): Unique presentation and a diagnostic dilemma. Ocul Immunol Inflamm 2022;00:1–4.
14. Mazzeo TJ, Sena NB, Motta MM, Curi AL. Diffuse unilateral subacute neuroretinitis evolving with submacular granuloma. Ocul Immunol Inflamm 2021;29:90–94.
15. Ramirez KK, Pizzimenti J, Wong-Powell J, Rabin J. Presumed diffuse unilateral subacute neuroretinitis: A review supported by a unique case. Optom Clin Pract 2019;1:10–26.
16. Saluja G, Joshi HK, Takkar B, Venkatesh P. Ultrawide field imaging with navigable magnifier for diagnosis of diffuse unilateral subacute neuroretinitis. BMJ Case Rep 2017;1–2.
17. Sodhi S, Golding J, Mandelcorn ED, Boggild AK, Choudhry N. Enface vitreous OCT ‘worm holes’: A novel finding in a patient with diffuse unilateral subacute neuroretinitis (DUSN). Am J Ophthalmol Case Reports 2021;23:101112.
18. Vianna RNG, G Onofre, V Ecard. et al. Indocyanine green angiography in diffuse unilateral subacute neuroretinitis. Eye 2006;20:1113–1116.
19. Gomes AH, Garcia CA, Segundo P, Garcia F, Garcia AC. Optic coherence tomography in a patient with diffuse unilateral subacute neuroretinitis. Arq Bras Oftalmol 2009;72:185–188.
20. Kalevar A, Jumper JM. Optical coherence tomography angiography of diffuse unilateral subacute neuroretinitis. Am J Ophthalmol Case Reports 2017;7:91–94.
21. Souza EC, Casella AMB, Nakashima Y, Monteiro ML R. Clinical features and outcomes of patients with diffuse unilateral subacute neuroretinitis treated with oral albendazole. Am J Ophthalmol 2005;140:437–445.
22. Amaral C, Jimenez H, Davila P, Ulloa-Padilla JP, Guiot HM, Oliver AL. Short-term oral albendazole therapy for diffuse unilateral subacute neuroretinitis: A case report. Am J Ophthalmol Case Reports 2021;22:101054.