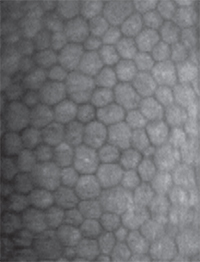
The endothelial layer is often considered a one-trick pony; its job is to maintain stromal deturgescence by regulating—usually limiting—flow of fluid from the aqueous humor into the corneal stroma. Composed of a single layer of flat hexagonal cells lining Descemet’s membrane, corneal endothelial cells form the interface between the cornea and the aqueous humor-filled anterior chamber. At just ~5 µm thick, the CE is the thinnest of the cellular corneal layers. The CE cells actively pump water from the stroma to the aqueous humor, maintaining a flow that balances the fluid leak through the extracellular space, and thus preserving the optical clarity of the cornea.
On average, the human cornea has an endothelial cell density in the range of 5,000 to 6,000 cells/mm2 at birth; that number decreases by 40 to 50 percent by adulthood2 at a cell loss of about 0.6 percent per year.1 Stromal edema typically appears when cell density decreases to between 400 and 700 cells/mm2. Why the cell loss? Under normal conditions, CE cells die at a rate comparable to other endothelial cells, however; they enter a state of apparent senescence early in life. The predominant compensatory response to cell loss is cell spreading and migration for maintenance of a uniform endothelial monolayer. If cell loss is accelerated, there comes a breaking point where the density of remaining cells cannot maintain stromal deturgescence and transparency.
|
To Divide or Not to Divide
Technically, it is not accurate to say that CE cells can’t divide. They can, but they don’t; a combination of cellular roadblocks freezes them in a well-differentiated state in the G1 phase of cell division. For many of the environmental and cellular regulators of cell division, signaling pathways converge on one or more of the kinases in the cyclin family. Cyclins function as gatekeepers in the transition between the phases of mitosis. For example, inhibition of cyclin E is thought to underlie the phenomenon of contact-mediated growth inhibition exhibited by many cells, including CE cells. Evidence from studies in both human CE and in animal models points to one cyclin inhibitor, a protein designated p27Kip1, in contact-mediated cell cycle arrest. Expression of p27Kip1 exhibits a twentyfold increase when cultured CE cells transition from sub-confluent to confluent.5,6 These same cultures can be induced to divide when cell-cell contacts are disrupted, and under these conditions p27Kip1 expression drops significantly.
Another factor that suppresses proliferation of CE cells is transforming growth factor beta 2, a cytokine found in relatively high levels in the aqueous humor; a number of studies have shown that TGF-β2 can suppress expression of key cyclins while also acting to maintain high levels of p27Kip1.6 These and perhaps other inputs act to prevent CE proliferation under normal physiological conditions.
Contrary to the long-standing notion of CE senescence, many recent reports suggest that CE cell division and proliferation can occur under specific conditions.7,8 For example, one group used a rat model of bullous keratopathy to show that peripheral CE cells divide and migrate toward the central endothelium, but cells in the central region do not.7 Other studies aimed at releasing CE cells from mitotic arrest have met with modest success and have established that the age is an important criterion; CE cells from younger donors have a much greater proliferative capacity than those from older donors.2,9
Predicting Endothelial Failure
Endothelial disorders are relatively common; recent estimates suggest 5 percent of individuals over 40 will experience some degree of Fuchs’ endothelial corneal dystrophy.2 Perhaps more important, patients with FECD who undergo surgical procedures for cataracts or glaucoma face an increase in the potential for endothelial dysfunction resulting from surgical trauma. Recent efforts have explored approaches to minimize this risk. For example, a 2013 study comparing torsional and longitudinal phacoemulsification showed that when less ultrasonic energy is used, the risk of endothelial sequela is reduced.10 But despite continuous refinement of surgical techniques, a modest perturbation of the CE can initiate an apoptotic cascade, accelerating the rate of cell death.
Even with these refined protocols to reduce the potential for adverse post-surgical CE outcomes, a different strategy may be required for patients already diagnosed with FECD who are also in need of cataract surgery. These patients face a complicated treatment outlook that often includes both cataract removal and eventual corneal transplant. While there has been significant progress in techniques used for endothelial transplant (see the September 2014 issue of Review for several excellent discussions of various methods for corneal transplantation) it’s clear that there is a need for non-surgical options to prevent or reverse CE cell loss.11
A significant advance came with the genome-wide association studies of FECD that identified several single risk alleles with unusually high odds ratios, confirming the strong genetic component of the disease.12-14 The association between FECD and single-sequence variants in the transcription factor 4 gene yielded odds ratios of 5.5, and those with multiple risk alleles had odds ratios of 30. It’s thought that TF4 may be involved in the differentiation of peripheral CE cell proliferation or in a limbal stem cell’s transition to an endothelial progenitor cell. Either case means the potential for endothelial regeneration is lost or impaired in individuals that carry TF4 variants.
|
Future Endothelial Therapies
While there is a significant body of research on potential therapies for CE diseases, most of this work has focused on in vitro studies or trials using animal models. The ultimate goal of these efforts has been the targeted prevention of endothelial cell death following various surgical procedures. Using a number of different protocols and experimental designs, comparisons of cell explants or cultured cells from FECD patients with control CE cells has yielded a general consensus that FECD cells are more sensitive to oxidative stress than control cells.15-17 This means that patients with one or more of the predisposing genetic traits linked to FECD are more vulnerable to triggers that initiate generation of reactive oxygen species, such as trauma or inflammation. There are likely additional factors contributing to increases in CE cell death in those with FECD: Even without the provocation of peroxide or some other oxidative stressor, FECD explants exhibit an average of 10 times the rate of apoptosis seen in control cells.17 Oxidative stress in FECD can also lead to apoptosis via effects on mitochondrial function, further supporting the idea that the molecular defect in FECD is an inability to respond appropriately to oxidative stress.18
As a proof of principle, a number of studies have demonstrated that classical anti-oxidants can mitigate CE cell loss under conditions of stress, even in cells from FECD subjects. A study published in 2013 followed this logic and examined the effects of sulforaphane, a naturally occurring isothiocyanate found in cruciferous vegetables that has been shown to activate endogenous anti-oxidative protective pathways.19 Interestingly, sulforaphane acts on a specific pathway (the Nrf2) that is known to be down-regulated in FECD. Treatment of ex vivo corneas from FECD subjects with sulforaphane prior to peroxide exposure normalized the response, suggesting that this or similar compounds may be a potential treatment for prevention of CE cell loss. One such compound, RTA-408,20 (Reata Pharmaceuticals; Irving, Texas) is currently in a Phase II trial for prevention of endothelial cell loss following cataract surgery.21
A completely different therapeutic strategy is exemplified by a new study testing Rho-associated kinase inhibitors as stimulators of endothelial cell proliferation.22 This approach is aimed at the endogenous pathways that limit CE cell growth; by altering cell adhesion, ROCK inhibitors attenuate cell-contact growth inhibition. This mechanism hasn’t been clearly established, but what is clear is that endothelial wound healing is accelerated by treatments mediated, at least in part, by new cell growth rather than by prevention of cell death.
These examples demonstrate that there are at least two distinct approaches that we may adopt in our efforts to preserve corneal endothelial cell function. Whether it’s by preventing cell death or, alternately, stimulating growth of new corneal endothelial cells, the overall function of the endothelial layer can be maintained. Ideally these efforts will lead to a reduction or elimination of the need for most corneal transplants. The benefits of these new therapies will be clear for all to see. REVIEW
Dr. Abelson is a clinical professor of ophthalmology at Harvard Medical School. Dr. McLaughlin is a medical writer at Ora Inc.
1. Edelhauser HF. The balance between corneal transparency and edema: The Proctor Lecture. Invest Ophthalmol Vis Sci 2006;47:1755–67.
2. Joyce NC. Proliferative capacity of corneal endothelial cells. Exp Eye Res 2012;95:1:16–23.
3. Adamis AP, Filatov V, Tripathi BJ, Tripathi RC. Fuchs’ endothelial dystrophy of the cornea. Surv Ophthalmol 1993;38:149-68.
4. Wagner M, Hampel B, Bernhard D, Hala M, Zwerschke W, Jansen-Dürr P. Replicative senescence of human endothelial cells in vitro involves G1 arrest, polyploidization and senescence-associated apoptosis. Exp Gerontol 2001;36:8:1327-47.
5. Yoshida K, Kase S, Nakayama K, et al. Involvement of p27KIP1 in the proliferation of the developing corneal endothelium. Invest Ophthalmol Vis Sci 2004;45:2163–2167.
6. Kim TY, Kim WI, Smith RE, Kay ED. Role of p27(Kip1) in cAMP- and TGF-beta2-mediated anti-proliferation in rabbit corneal endothelial cells. Invest Ophthalmol Vis Sci 2001;42:3142–3149.
7. Bredow L, Schwartzkopff J, Reinhard T. Regeneration of corneal endothelial cells following keratoplasty in rats with bullous keratopathy. Mol Vis 2014;20:683-90.
8. He Z, Campolmi N, Gain P, et al. Revisited microanatomy of the corneal endothelial periphery: New evidence for continuous centripetal migration of endothelial cells in humans. Stem Cells 2012;30:2523–2534.
9. Polisetti N, Joyce NC. The culture of limbal stromal cells and corneal endothelial cells. Methods Mol Biol 2013;1014:131-9.
10. Doors M, Berendschot TT, Touwslager W, Webers CA, Nuijts RM. Phacopower modulation and the risk for postoperative corneal decompensation: A randomized clinical trial. JAMA Ophthalmol 2013;131:11:1443-50.
11. Siu GD, Young AL, Jhanji V. Alternatives to corneal transplantation for the management of bullous keratopathy. Curr Opin Ophthalmol 2014;25:4:347-52.
12. Baratz KH, Tosakulwong N, Ryu E, et al. E2-2 protein and Fuchs’ corneal dystrophy. N Engl J Med 2010;363:1016-24.
13. Krachmer JH, Purcell JJ Jr, Young CW, Bucher KD. Corneal endothelial dystrophy: A study of 64 families. Arch Ophthalmol 1978;96:2036-9.
14. Wright AF, Dhillon B. Major progress in Fuchs’ corneal dystrophy. N Engl J Med 2010;363:1072-1075.
15. Azizi B, Ziaei A, Fuchsluger T, Schmedt T, Chen Y, Jurkunas UV. p53-regulated increase in oxidative-stress-induced apoptosis in Fuchs’ endothelial corneal dystrophy: A native tissue model. Invest Ophthalmol Vis Sci 2011;52:13:9291-7.
16. Jurkunas UV, Bitar MS, Funaki T, Azizi B. Evidence of oxidative stress in the pathogenesis of Fuchs’ endothelial corneal dystrophy. Am J Pathol 2010;177:5:2278-89.
17. Borderie VM, Baudrimont M, Vallée A, Ereau TL, Gray F, Laroche L. Corneal endothelial cell apoptosis in patients with Fuchs’ dystrophy. Invest Ophthalmol Vis Sci 2000;41:9:2501-5.
18. Czarny P, Seda A, Wielgorski M et al. Mutagenesis of mitochondrial DNA in Fuchs’ endothelial corneal dystrophy. Mutation Research Fundam Mol Mech Mutagen 2014;760:42–47.
19. Ziaei A, Schmedt T, Chen Y, Jurkunas UV. Sulforaphane decreases endothelial cell apoptosis in Fuchs’ endothelial corneal dystrophy: A novel treatment. Invest Ophthalmol Vis Sci 2013;54:10:6724-34.
20. Reisman SA, Lee CY, Meyer CJ, Proksch JW, Sonis ST, Ward KW. Topical application of the synthetic triterpenoid RTA 408 protects mice from radiation-induced dermatitis. Radiat Res 2014;181:5:512-20.
21.https://clinicaltrials.gov/ct2/results?term=NCT02128113 accessed 9 Oct 2014.
22. Koizumi N, Okumura N, Ueno M, Kinoshita S. New therapeutic modality for corneal endothelial disease using rho-associated kinase inhibitor eye drops. Cornea 2014;33:S25-S31.