The gut microbiome consists of a wide collection of archaea, eukaryotes, viruses and bacteria that play significant roles in human health and disease. Housed in the gastrointestinal tract and shaped by environmental/lifestyle factors, including geography, diet and medications, these commensal microorganisms are directly involved in numerous physiological functions, including nutrition, host immunity, drug metabolism and endocrine signaling.1 Yet, despite the microbiome’s overwhelming presence, our understanding of its dynamic interactions—particularly in diseased states—has only recently begun to grow.
The acceleration of next-generation analysis techniques has revealed direct connections between the microbiome and various disease pathologies. Improper microbiota composition and function (gut dysbiosis) has been linked with neurological, cardiovascular, respiratory and metabolic diseases, among others.2–5 These axes extend to the eye as well. Evidence has connected the microbiome with various retinal diseases, including age-related macular degeneration, diabetic retinopathy, retinopathy of prematurity, retinal artery occlusion and retinal dystrophies.6–11
In this review, we’ll examine the current knowledge surrounding the gut microbiota’s role in these retinal diseases in order to foster a better understanding of the various diseases’ mechanisms, and to potentially develop more targeted preventive and therapeutic interventions.
Age-Related Macular Degeneration
 |
|
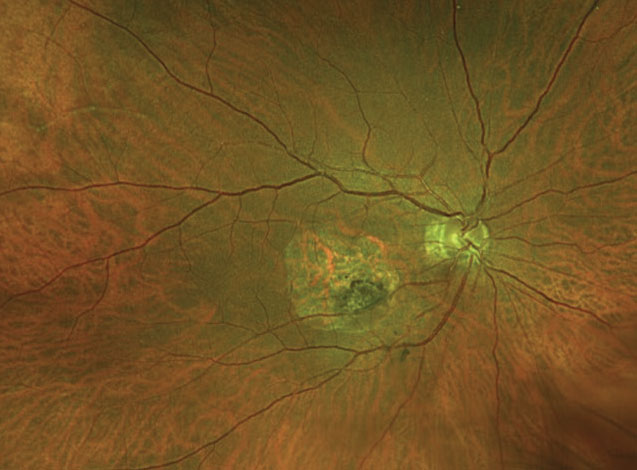
Figure 1. Right fundus with advanced dry AMD. The gut microbiome may play a key role in the impact of diet on AMD. |
As is commonly known, age-related macular degeneration has both exudative and non-exudative subtypes.12 Development of AMD depends on a number of risk factors, including age, genetic susceptibility and environmental influences such as diet and smoking. These components subsequently drive AMD pathogenesis through inflammation, oxidative stress and aberrant neovascularization. Although integrating these heterogeneous risk factors and mechanisms remains challenging, recent human and animal studies suggest that the gut microbiome plays a key role in reconciling the impact of these components.
Studies of gut dysbiosis have revealed significant changes in bacterial composition and diversity between states of health and AMD. Comparing a cohort of human neovascular AMD (nAMD) and control patients, Bern, Switzerland’s Martin Zinkernagel, MD, and colleagues identified compositional and functional variations between the groups’ intestinal microbiomes.6 Among the results, AMD patients were found to be enriched in the genus Oscillibacter, a microbial population implicated in high fat diets (HFD) and increased gut permeability. Furthermore, AMD patients were found to have an increase in the ratio of Firmicutes to Bacteroidetes—a known hallmark of obesity and a factor associated with exacerbation of choroidal neovascularization.13,14 In concordance with human studies, increased Firmicutes was also found in mice deficient in complement factor C3, a factor associated with AMD and negative retinal function.15–17 This was further supported by Changsha, China’s Yun Li, MD, and colleagues, who identified changes in the fecal microbiota of CNV mouse models, including altered metabolites in bile acid biosynthesis and elevations in proinflammatory bacteria.18
The distinct microbial profiles identified in preclinical and clinical models of AMD highlight a link between the microbiome and eye health. Through our and others’ work, this connection has been further reaffirmed by studies of diet-induced gut dysbiosis. In wild-type mice, Tuft’s University’s Sheldon Rowan, PhD, and his group found that administering high-glycemic-index diets promoted a pathogenesis similar to non-exudative AMD, including photoreceptor degeneration, sub-RPE membranous debris and lipofuscin accumulation.19 These effects were arrested or even reversed if mice were switched to lower glycemic diets. Meanwhile, Quebec’s Elisabeth MMA Andriessen and co-workers studied the impact of HFD in a mouse model of CNV, demonstrating an increase in CNV growth in HFD subjects compared to their regular-diet counterparts.14 Importantly, subsequent administration of oral antibiotics resulted in persistent weight gain but slowed CNV progression. With weight gain uncoupled from other factors, this suggested that direct alterations in the gut microbiome, and not obesity, were responsible for the choroidal angiogenesis.
These results closely align with previous analyses of dietary patterns in the Age-Related Eye Disease Studies trial. Trial participants with greater consumption of Western-style diets, including red meat and high-fat dairy products, showed significantly higher odds of AMD progression compared to leaner vegetable-based diets.20 Subsequently, as a result of the AREDS and AREDS2 trials, a number of anti-oxidants were identified to reduce risk of AMD progression.21 Although the protective mechanisms of these compounds aren’t fully understood, the presence of specific intestinal microbiota are fundamental to the bioavailability of many of the compounds. Furthermore, the profiled enhancement in gut microbiota alpha diversity (species richness and evenness) following AREDS supplementation suggests an important therapeutic role for the gut microbiome.8
The impact of diet-induced gut dysbiosis on AMD extends to the transcriptional level as well. Using high-throughput RNA sequencing, our team profiled the retinal transcriptomes of GF, or germ-free (i.e., lacking a microbiota) mice.22 Compared to control counterparts, absence of the gut microbiome resulted in significant differentially expressed genes (DEGs) in the retina, including vascular endothelial growth factor, AMP-activated protein kinase (AMPK), and proliferator-activated receptor gamma coactivator 1-alpha (PGC1A)—all of which have been implicated in AMD pathogenesis through aberrant neovascularization and cellular toxicity.23–25 Furthermore, we’ve previously shown that HFD consumption alters the retinal transcriptome both in the absence and presence of the microbial organ, with a unique signature profiled in each group. Among the differences include a number of genes associated with AMD pathologies, including those involved in the complement cascade, coagulation cascade and retinal inflammation.26,27
Given the role of gut microbiota in regulating innate and adaptive immunity, evidence has emerged linking genetic susceptibility, gut microbiota and inflammatory disease processes.28,29 In relation to AMD specifically, Bern’s Denise Zysset-Burri, PhD, and colleagues recently showed that the bacteria Negativicutes was more abundant in patients with nAMD, and positively correlated with complement gene CFH, an AMD-risk allele.15 Moreover, AMD patient gut microbiomes showed microbial gene-enrichment in various purine signaling pathways, which have been implicated in several retinal neovascular diseases.30,31 Similarly, the Casey Eye Institute’s Phoebe Lin, MD, PhD, and co-authors showed that a calculated AMD risk score correlated with ARMS2 and CFH risk alleles, as well as inversely correlated with gut microbiome alpha diversity in AMD patients.32 While no causal relationship has yet been established, this finding raises interesting possibilities between gut dysbiosis, complement dysregulation and AMD.
Beyond the complement cascade, dysregulation of several immune cell types, including microglia, macrophages and T-cells, has been identified in individuals with AMD.33–35 Similarly, it’s well-established that the gut microbiome influences both local and systemic immunity, extending to peripheral systems.36–38 In part, it’s thought that metabolite signaling from gut microbiota alters immune homeostasis. GF mouse experiments demonstrate that absence of microbiota leads to global deficits in microglial composition and maturation, which can be partially restored with introduction of gut microbiota and their metabolic products such as short-chain fatty acids (SCFAs).14 Additional mouse studies have shown that gut dysbiosis alters permeability of the intestinal epithelium, resulting in chronic, system inflammation with associated elevation of IL-6, TNF-α and IL-1β.39
These changes in gut microbiota have demonstrated far-reaching effects in retinal tissue. Yokahama, Japan’s Yuji Morita and colleagues demonstrated that administering the probiotic Lactobacillus paracasei KW3110 in aging mice reduced proinflammatory cytokine production in macrophages and age-related loss of retinal cells.40 They then showed that L. paracasei KW3110 reduced photoreceptor degeneration in a mouse model of light-induced retinopathy and was associated with an increased shift in M2-like macrophages, which is considered an anti-inflammatory phenotype. These studies demonstrate that alterations in gut microbiota can affect systemic immunity and inflammation. Collectively, these studies of the gut microbiome highlight the exciting yet complex relationship between diet, gut dysbiosis and AMD pathogenesis, necessitating further studies.
Diabetic Retinopathy
 |
|
Figure 2. Patient with a quiescent PDR. Given the metabolic nature of diabetes, researchers say it has many associations with the gut microbiome. |
Approximately one-third of individuals diagnosed with diabetic retinopathy have vision-threatening disease.41 The number of adults with DR was estimated to be 103.1 million worldwide in 2020, with a projected increase to 160.5 million adults in 2045.42 As it correlates with diabetes status, risk factors of DR include hyperglycemia, hypertension, smoking and dyslipidemia.43 The chronic hyperglycemia causes shifts in cellular metabolism and the release of growth factors, resulting in sorbitol accumulation, oxidative stress, activation of protein kinase C, and increased non-enzymatic protein glycation. These pathologic changes impair visual function by means of capillary leakage, occlusion and sequelae of retinal ischemia, including neovascularization and vitreous hemorrhage.44
Given the metabolic and inflammatory nature of diabetes, many associations exist between the gut microbiome and the prevalence and progression of type 2 diabetes mellitus (T2DM).45,46 Interestingly, several studies have identified independent changes in the gut microbiome between individuals with diabetes and those with concurrent DR. For instance, one study found that while both groups have different gut microbial compositions relative to healthy controls, such as increased levels of Bifidobacterium and Lactobacillus, the DR group had lower levels of Pasteurellaceae and Firmicutes compared to the non-DR group.47 Similarly, Hyderabad, India’s Taraprasad Das, MD, and his group reported that individuals with T2DM compared to those with T2DM and DR had significant differences in gut microbiome composition at the genera level.48 They further noted the DR group had decreased Lactobacillus and Actinobacteria, and increased Shigella, which together suggest a potential decline in anti-inflammatory and probiotic bacteria.
There are several proposed mechanisms for how gut microbiota may impact DR pathophysiology. In addition to changes that occur locally in the gastrointestinal tract, alterations in gut microbiota may influence host immunity and metabolism systemically. For instance, elevation of bacterial products, such as lipopolysaccharide (LPS), has been shown to exacerbate retinal endothelial injury in mice with pre-existing risk factors such as hyperglycemia.49 Furthermore, differential levels of metabolites processed by gut microbiota, including SCFAs, bile acids and lipids have been observed in patients with T2DM compared to healthy controls, which may also contribute to DR pathobiology.50 One study in patients with diabetes showed that those with concurrent PDR had different gut bacteria compositions with significant differences in fecal metabolites, specifically in pathways of arachidonic acid and microbial metabolism.51
In diabetic db/db mice, intermittent fasting was shown to alter gut microbiota composition, increasing Firimicutes and decreasing Bacteroidetes and Verrucomicrobia, with an associated reduction in clinical markers of DR such as acellular capillaries and leukocyte infiltration.52 Elevation of the bile acid metabolite tauroursodeoxycholate (TUDCA), a neuroprotective molecule, was observed, which was consistent with Firmicutes’ ability to modulate bile acid metabolism. Another microbial metabolite associated with DR in patients is trimethylamine-N-oxide, which is derived from dietary choline.53
Preclinical experiments have begun investigating gut microbiota modulation to target DR. Administering recombinant Lactobacillus paracasei in mice with DR has shown to reduce retinal capillary cell loss, inflammatory cytokine production and gliosis.54,55 While no gut microbiome-centered interventions have been tested specifically for DR in humans, promising results are seen in clinical trials involving diet modulation and fecal microbiota transplantation, resulting in controlled blood sugar and insulin production, thereby potentially affecting DR development.56,57
In conclusion, visual impairment caused by DR is closely tied with diabetes pathophysiology, and together they share associated changes in gut microbiota composition and metabolic pathways, which ultimately can affect systemic metabolism and inflammation. Studies also show distinct microbiota profiles between patients with diabetes alone compared to patients with concurrent DR, which may suggest unique contributions of gut microbiota in DR pathology and require further investigation.
Retinopathy of Prematurity
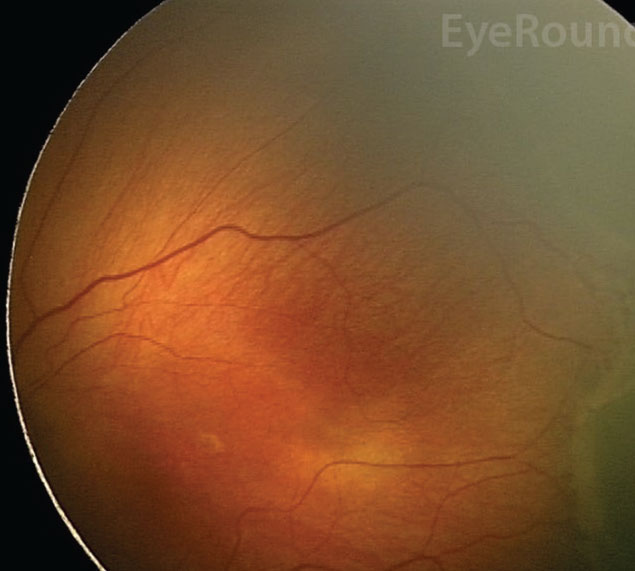 |
|
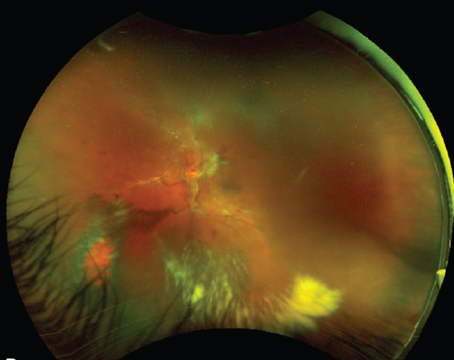
Figure 3. Stage 3 retinopathy of prematurity. Preliminary studies found gut dysbiosis in infants with ROP. Image: Mai AP, Scruggs BA, Kemp PS. Retinopathy of prematurity. Accessed April 11, 2022. https://webeye.ophth.uiowa.edu/eyeforum/cases/286-retinopathy-of-prematurity.htm |
Classically, the pathogenesis of this disease of premature and low-weight infants58,59 occurs in two successive phases: an ischemic phase in which normal retinal vasculature fails to develop; followed by a vasoproliferative phase in which abnormal neovascularization occurs.
Various diseases of prematurity, including ROP, have been associated with microbial imbalances. For instance, preterm infants may have less microbiome diversity and potentially more pathogenic strains of bacteria.60–63 Furthermore, overall gut microbiome composition is heavily influenced by gestational age (GA) at birth, mode of delivery (i.e., Cesarean section or vaginal birth) and infant diet (i.e., breast milk or formula fed), highlighting various stages at which dysbiosis may occur.64–68
We recently analyzed fecal samples from preterm infants with type I ROP needing treatment and similarly-matched high-risk preterm infants without ROP. Infants with severe ROP had significant enrichment of the bacteria family Enterobacteriaceae at 28 weeks postmenstrual age, which includes pathogens such as Escherichia coli, Salmonella and Shigella.69 Meanwhile, the microbiota of infants without ROP showed enrichment of metabolic pathways involved in oxidative phosphorylation, amino acid synthesis and degradation, and bacterial metabolites known to be beneficial to human health.69 In a similar study conducted in Australia, fecal samples were analyzed from preterm infants (born <32 weeks GA and weighing <1,500 g) who received probiotics while hospitalized in the intensive care unit. Upon admission, infants with ROP had a lower diversity of organisms and a greater abundance of Staphylococcus species.70 Though preliminary, the results of these two studies suggest that early gut dysbiosis with overpopulation of pathogenic bacteria and consequential poor development of metabolic pathways may contribute to ROP development in prenatal infants.7,69,70
These findings are supported by our knowledge that important risk factors for ROP also influence gut microbiome composition. Maternal age, smoking status, gestational diabetes and hypertension during pregnancy have all been associated with ROP and neonatal gut microbiome alterations.71 Also, necrotizing enterocolitis and neonatal sepsis are independent risk factors for ROP, and also are associated with changes in the neonatal gut microbiome.71 At the diet level, human breast milk is known to protect against ROP.72,73 This may be mediated by increasing IGF-1 levels, which are regulated by the gut microbiome and serve as a protective factor against ROP’s development.69,71,72
Central Retinal Artery Occlusion
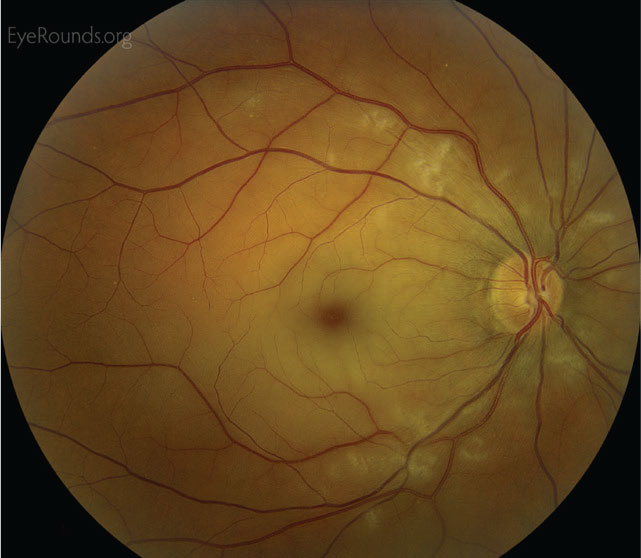 |
|
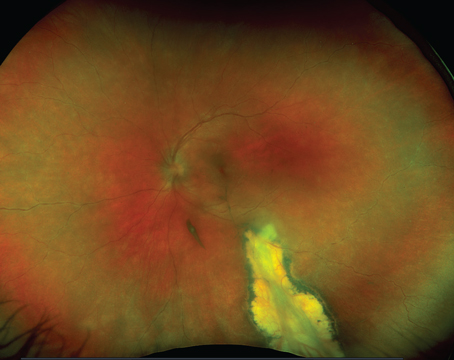
Figure 4. An acute CRAO. Patients with RAO have demonstrated alterations in their gut microbiomes. Image: Phillips D, Starkweather A. Central retinal artery occlusion. Accessed April 11, 2022. https://webeye.ophth.uiowa.edu/eyeforum/atlas/pages/CRAO/index.htm |
In non-arteritic retinal artery occlusion a thromboembolic plaque occludes either the central retinal artery or a branch retinal artery, leading to vision loss from inner retinal ischemia, atrophy and possible neovascularization.74 Given that RAO occurs in conjunction with systemic atherosclerosis, RAO risk factors are similar and include smoking, diet, exercise, hypertension and hypercholesterol.75,76 Stroke, cardiovascular disease and atherosclerosis have all been associated with alterations in the gut microbiome.77,78 Although the exact relationship is unknown, the gut microbiome influences circulating levels of lipids, insulin resistance, adipocyte fat storage and systemic inflammation.79–83 Furthermore, studies show that bacterial DNA is present within atherosclerotic plaques and that the source may be a dysbiotic gut microbiome.84,85
Only one study to date has investigated the relationship between the gut microbiome and RAO development.86 Comparing the gut metagenomes of patients with non-arteritic RAO and matched healthy controls, patients with RAO had significant alterations in their gut microbiota composition, suggesting a role of gut dysbiosis in RAO pathogenesis. Additionally, levels of trimethylamine-N-oxide (TMAO), a pro-atherogenic, gut-derived metabolite that interferes with cholesterol transport, was increased in patients with RAO.86–88 Collectively, these findings suggest that an important but unknown relationship could exist between the gut microbiome and the development of RAO and other atherosclerotic diseases.
Retinal Dystrophies
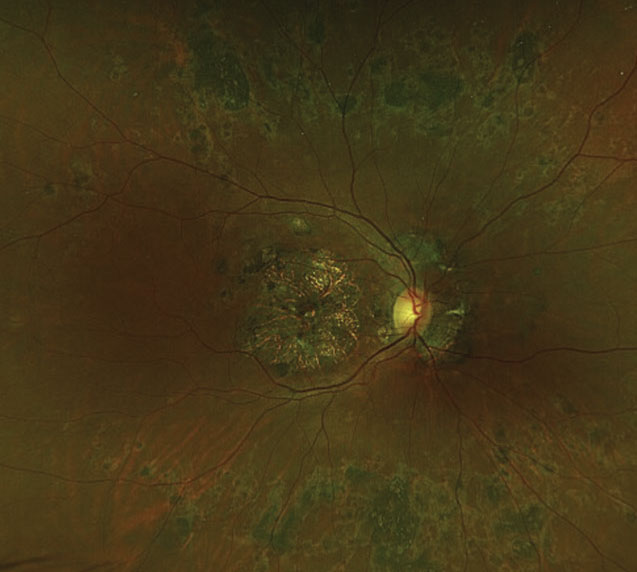 |
|
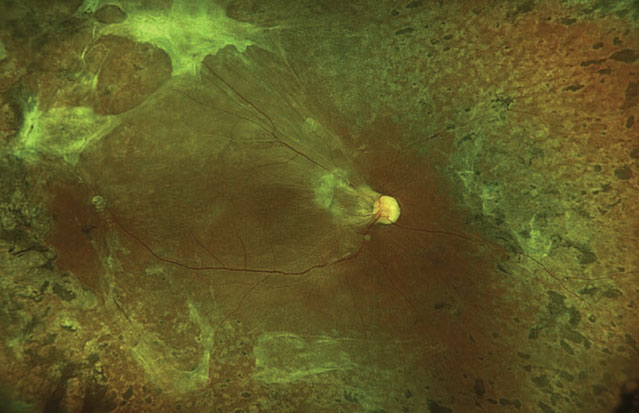
Figure 5. Fundus photo of a patient with a presumed retinal dystrophy with positive USH2A and PEX16 mutations, demonstrating a large central area of atrophy in the macula and chorioretinal lesions. A mouse model of retinal dystrophy showed a pattern of gut dysbiosis. |
A limited but emerging body of research has associated gut microbiome composition with retinal dystrophies, particularly retinitis pigmentosa. In a mouse model of RP, researchers noted a pattern of gut dysbiosis correlated with classic markers of RP functional decline. Decreases in visual acuity and retinal responsiveness to light were accompanied by decreases in microbiome diversity, enrichment in Bacteroides caecimuris, and a complete absence of Rikenella spp., Muribaculaceace spp. and Bacilli spp.9 Although the significance of these compositional changes has yet to be determined, these shifts implicate a link between RP and dysbiosis.
Previous studies reveal possible explanations for these findings. The well-documented inflammatory nature of RP strongly suggests that concomitant changes between microbial composition and RP progression are mediated partly by an inflammatory response.89–91 It’s been shown that LPS-induced peripheral inflammation exacerbates inflammatory and apoptotic states seen in P23H rats, which is another RP model.92 This resulted in not only a reduction in cone photoreceptors, but also an increase in microglial activation and gliosis. Related to this, human patients with intestinal barrier dysfunction demonstrated a correlative increase in plasma LPS-positive bacterial extracellular vesicles.93 This further supports the notion that gut dysbiosis can lead to various systemic inflammatory and pathogenic effects and may modulate phenotypical severity of retinal dystrophy.
A recent study shed further light on the purported mechanistic relationship between gut dysbiosis and RP-induced degeneration. RP mice fed a HFD for two to three weeks had accelerated pathologic retinal degeneration as measured by retinal responsiveness, photoreceptor degeneration and second-order neuron connectivity.94 These changes were associated with a similar reduction in gut microbiome diversity, as well as increases in pro-inflammatory bacteria and inflammatory modulators including GSK3β, STAT3 and NF-κB. This degeneration was further exacerbated by increased oxidative stress. Thus, these changes highlight the detrimental impact of a Western HFD in retinal dystrophies and the possible role of gut dysbiosis in this progression.
The idea that diet impacts the modulation and progression of retinal diseases such as RP is not new. A previous study showed that RP patients with high omega-3 intake (≥ 0.20 g/day) had significantly slower rates of decline in distance and retinal visual acuities compared to control counterparts.95 Additionally, ketogenic diets were shown to promote neuroprotection and enhanced visual function in mouse models of RP.96 Collectively, these findings, alongside previous literature, support the need to incorporate diet awareness into patient education and disease management. However, further research is needed to distill the precise mechanistic role of the microbiome in retinal dystrophies.
In conclusion, over the past several decades, there’s been an increased interest in how our microbial organ contributes to health and disease. Indeed, gut dysbiosis has been strongly associated with a host of local gastrointestinal and distant organ system issues, including cardiovascular, pulmonary and central nervous systems issues. Recent studies have been developing the notion of a gut microbiome-retina axis, in which the gut microbiome could play a fundamental role in retinal disease.
In this review, we discussed how the gut microbiome is associated with AMD, RAO, ROP, DR and retinal dystrophies, as well as how dysbiosis may contribute to their pathogeneses. While the exact mechanisms aren’t fully understood, the impact of the gut microbiome in altering systemic inflammation, host immunity and metabolic signaling is significant, and may well directly contribute to ocular health and disease. Further studies on the gut microbiome-retina axis may not only improve our understanding of retinal diseases, but also help identify new screening tools and therapeutics that will advance clinical care.
Corresponding author:
Dimitra Skondra, MD, PhD
Email: dskondra@bsd.uchicago.edu
5841 S. Maryland Avenue, S426m MC2114, Chicago, IL 60637
Office: 773-702-3937
Fax: 773-702-0830
1. Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 2021;19:1:55-71.
2. Parodi B, Kerlero de Rosbo N. The gut-brain axis in multiple sclerosis. Is its dysfunction a pathological trigger or a consequence of the disease? Front Immunol 2021;12:718220.
3. Trøseid M, Andersen GØ, Broch K, Hov JR. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine 2020;52:102649.
4. Chunxi L, Haiyue L, Yanxia L, Jianbing P, Jin S. The gut microbiota and respiratory diseases: New evidence. J Immunol Res 2020;2020:2340670.
5. Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest 2019;129:10:4050-4057.
6. Zinkernagel MS, Zysset-Burri DC, Keller I, et al. Association of the intestinal microbiome with the development of neovascular age-related macular degeneration. Sci Rep 2017;7:40826.
7. Nadeem U, Boachie-Mensah M, Zhang J, Skondra D. Gut microbiome and retinal diseases: An updated review. Current Opinion in Ophthalmology. Published online February 23, 2022.
8. Lin P, McClintic SM, Nadeem U, Skondra D. A review of the role of the intestinal microbiota in age-related macular degeneration. Journal of Clinical Medicine 2021;10:10:2072.
9. Kutsyr O, Maestre-Carballa L, Lluesma-Gomez M, Martinez-Garcia M, Cuenca N, Lax P. Retinitis pigmentosa is associated with shifts in the gut microbiome. Sci Rep 2021;11:1:6692.
10. Skondra D, Rodriguez SH, Sharma A, Gilbert J, Andrews B, Claud EC. The early gut microbiome could protect against severe retinopathy of prematurity. J AAPOS 2020;24:4:236-238.
11. Beli E, Yan Y, Moldovan L, et al. Restructuring of the gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in db/db mice. Diabetes 2018;67:9:1867-1879.
12. Rein DB, Wittenborn JS, Zhang X, et al. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol 2009;127:4:533-540.
13. Aoun A, Darwish F, Hamod N. The influence of the gut microbiome on obesity in adults and the role of probiotics, prebiotics, and synbiotics for weight loss. Prev Nutr Food Sci 2020;25:2:113-123.
14. Andriessen EM, Wilson AM, Mawambo G, et al. Gut microbiota influences pathological angiogenesis in obesity-driven choroidal neovascularization. EMBO Mol Med 2016;8:12:1366-1379.
15. Zysset-Burri DC, Keller I, Berger LE, et al. Associations of the intestinal microbiome with the complement system in neovascular age-related macular degeneration. npj Genom Med 2020;5:1:1-11.
16. Cashman SM, Desai A, Ramo K, Kumar-Singh R. Expression of complement component 3 (C3) from an adenovirus leads to pathology in the murine retina. Invest Ophthalmol Vis Sci 2011;52:6:3436-3445.
17. Hoh Kam J, Lenassi E, Malik TH, Pickering MC, Jeffery G. Complement component C3 plays a critical role in protecting the aging retina in a murine model of age-related macular degeneration. Am J Pathol 2013;183:2:480-492.
18. Li Y, Cai Y, Huang Q, et al. Altered fecal microbiome and metabolome in a mouse model of choroidal neovascularization. Frontiers in Microbiology 2021;12:2417.
19. Rowan S, Jiang S, Korem T, et al. Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc Natl Acad Sci U S A 2017;114:22:E4472-E4481.
20. Chiu CJ, Chang ML, Zhang FF, et al. The relationship of major American dietary patterns to age-related macular degeneration. Am J Ophthalmol 2014;158:1:118-127.e1.
21. Chew EY, Clemons TE, Agrón E, et al. Effect of omega-3 fatty acids, lutein/zeaxanthin, or other nutrient supplementation on cognitive function: The AREDS2 randomized clinical trial. JAMA 2015;314:8:791-801.
22. Nadeem U, Xie B, Movahedan A, et al. High throughput RNA sequencing of germ-free mouse retina reveals metabolic pathways involved in the gut-retina axis. bioRxiv. Published online November 14, 2020:2020.10.01.318949. doi:10.1101/2020.10.01.318949.
23. Veritti D, Sarao V, Soppelsa V, Danese C, Chhablani J, Lanzetta P. Managing neovascular age-related macular degeneration in clinical practice: Systematic review, meta-analysis, and meta-regression. J Clin Med. 2022;11:2:325.
24. Xu L, Kong L, Wang J, Ash JD. Stimulation of AMPK prevents degeneration of photoreceptors and the retinal pigment epithelium. PNAS 2018;115:41:10475-10480.
25. Felszeghy S, Viiri J, Paterno JJ, et al. Loss of NRF-2 and PGC-1α genes leads to retinal pigment epithelium damage resembling dry age-related macular degeneration. Redox Biol 2018;20:1-12.
26. Dao D, Xie B, Nadeem U, et al. High-fat diet alters the retinal transcriptome in the absence of gut microbiota. Cells 2021;10:8:2119.
27. Nadeem U, Xie B, Souza MD, et al. RNA sequencing reveals changes in mouse retinal transcriptome caused by high-fat diet induced gut dysbiosis. Investigative Ophthalmology & Visual Science 2021;62:8:2236.
28. Imhann F, Vich Vila A, Bonder MJ, et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut 2018;67:1:108-119.
29. Nissilä E, Korpela K, Lokki AI, et al. C4B gene influences intestinal microbiota through complement activation in patients with paediatric-onset inflammatory bowel disease. Clinical and Experimental Immunology 2017;190:3:394-405.
30. Hou XW, Wang Y, Pan CW. Metabolomics in age-related macular degeneration: A systematic review. Invest Ophthalmol Vis Sci 2020;61:14:13.
31. Liu K, Fang J, Jin J, et al. Serum metabolomics reveals personalized metabolic patterns for macular neovascular disease patient stratification. J Proteome Res 2020;19:2:699-707.
32. Lin P, McClintic SM, Nadeem U, Skondra D. A review of the role of the intestinal microbiota in age-related macular degeneration. J Clin Med 2021;10:10:2072.
33. Singh A, Subhi Y, Krogh Nielsen M, et al. Systemic frequencies of T helper 1 and T helper 17 cells in patients with age-related macular degeneration: A case-control study. Sci Rep 2017;7:1:605.
34. Cao X, Shen D, Patel MM, et al. Macrophage polarization in the maculae of age-related macular degeneration: A pilot study. Pathol Int 2011;61:9:528-535.
35. Combadière C, Feumi C, Raoul W, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest 2007;117:10:2920-2928.
36. Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012;489:7415:231-241.
37. Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012;3:1:4-14.
38. Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res 2020;30:6:492-506.
39. Andriessen EM, Wilson AM, Mawambo G, et al. Gut microbiota influences pathological angiogenesis in obesity‐driven choroidal neovascularization. EMBO Mol Med 2016;8:12:1366-1379.
40. Morita Y, Jounai K, Sakamoto A, et al. Long-term intake of Lactobacillus paracasei KW3110 prevents age-related chronic inflammation and retinal cell loss in physiologically aged mice. Aging (Albany NY) 2018;10:10:2723-2740.
41. Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2015;2:17.
42. Teo ZL, Tham YC, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology 2021;128:11:1580-1591.
43. Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol 2020;18:2:117-124.
44. Shukla UV, Tripathy K. Diabetic Retinopathy. In: StatPearls. StatPearls Publishing; 2022. Accessed February 19, 2022. http://www.ncbi.nlm.nih.gov/books/NBK560805/
45. Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012;490:7418:55-60.
46. Aron-Wisnewsky J, Warmbrunn MV, Nieuwdorp M, Clément K. Metabolism and metabolic disorders and the microbiome: The intestinal microbiota associated with obesity, lipid metabolism, and metabolic health-pathophysiology and therapeutic strategies. Gastroenterology 2021;160:2:573-599.
47. Huang Y, Wang Z, Ma H, et al. Dysbiosis and implication of the gut microbiota in diabetic retinopathy. Front Cell Infect Microbiol 2021;11:646348.
48. Das T, Jayasudha R, Chakravarthy S, et al. Alterations in the gut bacterial microbiome in people with type 2 diabetes mellitus and diabetic retinopathy. Sci Rep 2021;11:1:2738.
49. Vagaja NN, Binz N, McLenachan S, Rakoczy EP, McMenamin PG. Influence of endotoxin-mediated retinal inflammation on phenotype of diabetic retinopathy in Ins2 Akita mice. Br J Ophthalmol 2013;97:10:1343-1350.
50. Zhao L, Lou H, Peng Y, Chen S, Zhang Y, Li X. Comprehensive relationships between gut microbiome and faecal metabolome in individuals with type 2 diabetes and its complications. Endocrine 2019;66:3:526-537.
51. Ye P, Zhang X, Xu Y, Xu J, Song X, Yao K. Alterations of the gut microbiome and metabolome in patients with proliferative diabetic retinopathy. Frontiers in Microbiology 2021;12. Accessed February 20, 2022. https://www.frontiersin.org/article/10.3389/fmicb.2021.667632
52. Beli E, Yan Y, Moldovan L, et al. Restructuring of the gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in db/db mice. Diabetes 2018;67:9:1867-1879.
53. Liu W, Wang C, Xia Y, et al. Elevated plasma trimethylamine-N-oxide levels are associated with diabetic retinopathy. Acta Diabetol 2021;58:2:221-229.
54. Verma A, Xu K, Du T, et al. Expression of human ACE2 in lactobacillus and beneficial effects in diabetic retinopathy in mice. Mol Ther Methods Clin Dev 2019;14:161-170.
55. Verma A, Zhu P, Xu K, et al. Angiotensin-(1–7) expressed from lactobacillus bacteria protect diabetic retina in mice. Transl Vis Sci Technol 2020;9:13:20.
56. Groot P de, Nikolic T, Pellegrini S, et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut 2021;70:1:92-105.
57. Su L, Hong Z, Zhou T, et al. Health improvements of type 2 diabetic patients through diet and diet plus fecal microbiota transplantation. Sci Rep 2022;12:1:1152.
58. Chen J, Smith LEH. Retinopathy of prematurity. Angiogenesis 2007;10:2:133-140.
59. American Academy of Pediatrics Section on Ophthalmology, American Academy Of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus, et al. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131(1):189-195. doi:10.1542/peds.2012-2996
60. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5(1):4. doi:10.1186/s40168-016-0213-y
61. Dahl C, Stigum H, Valeur J, et al. Preterm infants have distinct microbiomes not explained by mode of delivery, breastfeeding duration or antibiotic exposure. Int J Epidemiol. 2018;47(5):1658-1669. doi:10.1093/ije/dyy064
62. Schwiertz A, Gruhl B, Löbnitz M, Michel P, Radke M, Blaut M. Development of the intestinal bacterial composition in hospitalized preterm infants in comparison with breast-fed, full-term infants. Pediatr Res. 2003;54(3):393-399. doi:10.1203/01.PDR.0000078274.74607.7A
63. Arboleya S, Sánchez B, Milani C, et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr. 2015;166(3):538-544. doi:10.1016/j.jpeds.2014.09.041
64. Zhuang L, Chen H, Zhang S, Zhuang J, Li Q, Feng Z. Intestinal microbiota in early life and its implications on childhood health. Genomics Proteomics Bioinformatics. 2019;17(1):13-25. doi:10.1016/j.gpb.2018.10.002
65. Chernikova DA, Koestler DC, Hoen AG, et al. Fetal exposures and perinatal influences on the stool microbiota of premature infants. J Matern Fetal Neonatal Med. 2016;29(1):99-105. doi:10.3109/14767058.2014.987748
66. Itani T, Ayoub Moubareck C, Melki I, et al. Establishment and development of the intestinal microbiota of preterm infants in a Lebanese tertiary hospital. Anaerobe. 2017;43:4-14. doi:10.1016/j.anaerobe.2016.11.001
67. Mshvildadze M, Neu J, Shuster J, Theriaque D, Li N, Mai V. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J Pediatr. 2010;156(1):20-25. doi:10.1016/j.jpeds.2009.06.063
68. Stewart CJ, Ajami NJ, O’Brien JL, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562(7728):583-588. doi:10.1038/s41586-018-0617-x
69. Skondra D, Rodriguez SH, Sharma A, Gilbert J, Andrews B, Claud EC. The early gut microbiome could protect against severe retinopathy of prematurity. J AAPOS. 2020;24(4):236-238. doi:10.1016/j.jaapos.2020.03.010
70. Westaway JAF, Huerlimann R, Kandasamy Y, et al. The bacterial gut microbiome of probiotic-treated very-preterm infants: changes from admission to discharge. Pediatr Res. Published online October 7, 2021:1-9. doi:10.1038/s41390-021-01738-6
71. Kim SJ, Port AD, Swan R, Campbell JP, Chan RVP, Chiang MF. Retinopathy of prematurity: A review of risk factors and their clinical significance. Surv Ophthalmol. 2018;63(5):618-637. doi:10.1016/j.survophthal.2018.04.002
72. Bharwani SK, Green BF, Pezzullo JC, Bharwani SS, Bharwani SS, Dhanireddy R. Systematic review and meta-analysis of human milk intake and retinopathy of prematurity: a significant update. J Perinatol. 2016;36(11):913-920. doi:10.1038/jp.2016.98
73. Fang JL, Sorita A, Carey WA, Colby CE, Murad MH, Alahdab F. Interventions to prevent retinopathy of prematurity: A meta-analysis. Pediatrics. 2016;137(4):e20153387. doi:10.1542/peds.2015-3387
74. Hayreh SS. Acute retinal arterial occlusive disorders. Prog Retin Eye Res. 2011;30(5):359-394. doi:10.1016/j.preteyeres.2011.05.001
75. The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251(3):351-364. doi:10.1001/jama.1984.03340270029025
76. Frost PH, Davis BR, Burlando AJ, et al. Serum lipids and incidence of coronary heart disease. Findings from the Systolic Hypertension in the Elderly Program (SHEP). Circulation. 1996;94(10):2381-2388. doi:10.1161/01.cir.94.10.2381
77. Benakis C, Brea D, Caballero S, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med. 2016;22(5):516-523. doi:10.1038/nm.4068
78. Kazemian N, Mahmoudi M, Halperin F, Wu JC, Pakpour S. Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome. 2020;8(1):36. doi:10.1186/s40168-020-00821-0
79. Fu J, Bonder MJ, Cenit MC, et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res. 2015;117(9):817-824. doi:10.1161/CIRCRESAHA.115.306807
80. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA. 2002;287(19):2570-2581. doi:10.1001/jama.287.19.2570
81. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480-484. doi:10.1038/nature07540
82. Armstrong EJ, Rutledge JC, Rogers JH. Coronary artery revascularization in patients with diabetes. Circulation. 2013;128(15):1675-1685. doi:10.1161/CIRCULATIONAHA.113.002114
83. Thevaranjan N, Puchta A, Schulz C, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21(4):455-466.e4. doi:10.1016/j.chom.2017.03.002
84. Koren O, Spor A, Felin J, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proceedings of the National Academy of Sciences. 2011;108(Supplement_1):4592-4598. doi:10.1073/pnas.1011383107
85. Lindskog Jonsson A, Hållenius FF, Akrami R, et al. Bacterial profile in human atherosclerotic plaques. Atherosclerosis. 2017;263:177-183. doi:10.1016/j.atherosclerosis.2017.06.016
86. Zysset-Burri DC, Keller I, Berger LE, et al. Retinal artery occlusion is associated with compositional and functional shifts in the gut microbiome and altered trimethylamine-N-oxide levels. Sci Rep. 2019;9(1):15303. doi:10.1038/s41598-019-51698-5
87. Li XS, Obeid S, Klingenberg R, et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38(11):814-824. doi:10.1093/eurheartj/ehw582
88. Schiattarella GG, Sannino A, Toscano E, et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J. 2017;38(39):2948-2956. doi:10.1093/eurheartj/ehx342
89. Noailles A, Maneu V, Campello L, Gómez-Vicente V, Lax P, Cuenca N. Persistent inflammatory state after photoreceptor loss in an animal model of retinal degeneration. Sci Rep. 2016;6(1):33356. doi:10.1038/srep33356
90. Yoshida N, Ikeda Y, Notomi S, et al. Clinical evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology. 2013;120(1):100-105. doi:10.1016/j.ophtha.2012.07.006
91. Olivares-González L, Velasco S, Campillo I, Rodrigo R. Retinal inflammation, cell death and inherited retinal dystrophies. Int J Mol Sci. 2021;22(4):2096. doi:10.3390/ijms22042096
92. Noailles A, Maneu V, Campello L, Lax P, Cuenca N. Systemic inflammation induced by lipopolysaccharide aggravates inherited retinal dystrophy. Cell Death Dis. 2018;9(3):1-18. doi:10.1038/s41419-018-0355-x.
93. Tulkens J, Vergauwen G, Van Deun J, et al. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut. 2020;69(1):191-193. doi:10.1136/gutjnl-2018-317726.
94. Kutsyr O, Noailles A, Martínez-Gil N, et al. Short-term high-fat feeding exacerbates degeneration in retinitis pigmentosa by promoting retinal oxidative stress and inflammation. PNAS. 2021;118(43). doi:10.1073/pnas.2100566118.
95. Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, Willett WC. Omega-3 intake and visual acuity in patients with retinitis pigmentosa on vitamin A. Arch Ophthalmol. 2012;130(6):707-711. doi:10.1001/archophthalmol.2011.2580.
96. Wert KJ, Velez G, Kanchustambham VL, et al. Metabolite therapy guided by liquid biopsy proteomics delays retinal neurodegeneration. EBioMedicine. 2020;52:102636. doi:10.1016/j.ebiom.2020.102636.