Technology can sometimes be a polarizing topic in health care. While many advancements have led to improved patient outcomes, there are also those that have introduced a new set of obstacles for physicians to overcome. Some technologies can even fit into both of these camps.
Take optical coherence tomography. Few ophthalmologists would argue its impact on the field. In its approximately three-decade existence, OCT has opened a window into the diagnosis and treatment of retinal and anterior segment diseases, and its continued evolution with the development of optical coherence tomographic angiography will likely only get better. However, OCT is not without its limitations, the most common of which are image artifacts. These artifacts can influence how data are interpreted and possibly make your determinations inaccurate.
We spoke with retina specialists about how to spot artifacts and what steps to take to reduce them.
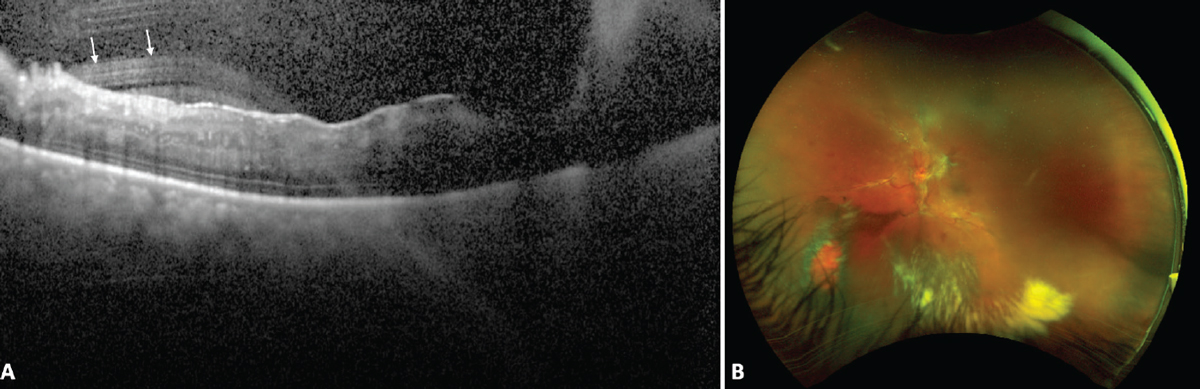 |
| Figure 1. Image artifact resulting in degraded OCT image due to vitreous hemorrhage in an eye with proliferative diabetic retinopathy. OCT (A) shows artifactual hyperreflective streaks in the vitreous (white arrows) and poor image quality due to the shadowing caused by the vitreous hemorrhage in the right half of the image resulting in poor visualization of the retinal layers. Fundus photograph (B) shows proliferative diabetic retinopathy with an old vitreous hemorrhage. Photo: Dilraj Grewal, MD. |
A Closer Look
Dilraj Grewal, MD, an ophthalmologist and retinal surgeon at Duke Eye Health in North Carolina, agrees that OCT has transformed the field of ophthalmology. “OCT is an essential component of our modern ocular exam. In fact, we can also consider it an ocular vital sign. For retinal patients, it’s an integral and indispensable part of evaluation and decision-making,” he says.
Keeping in mind how important OCT is, Dr. Grewal doesn’t take any scan at face value. “As with any kind of imaging modality, it’s very important to consider scan quality as an integral component of your decision-making because what you see is what you get. If your inputs aren’t accurate, which are impacted by scan quality, then your outputs or treatment decisions may not be accurate either,” he advises.
Nadia Waheed, MD, MPH, an associate professor at the Tufts University School of Medicine in Boston, says OCT provides incredibly dense information, with various forms of data output: linear (B) scans, through the area of interest/fovea; and volumetric scans, obtained through raster or cross-scanning of the macula. The information presented can be qualitative, i.e., identifying retinal pathologies; or quantitative, e.g., calculating retinal thickness.1
“OCT devices can acquire line scans, which are multiple acquisitions at exactly the same area. You then average those acquisitions to get better visualization,” Dr. Waheed says. “The other output is the macular cube scan, which takes multiple images of adjacent areas, most commonly centered on the fovea. When you’re interpreting OCT qualitatively, it’s really important to look at the entire cube and not just one picture from the cube, because then you could easily miss things.”
Lisa Olmos de Koo, MD, MBA, associate professor of ophthalmology in the vitreoretinal surgery division at the University of Washington in Seattle, finds the cube scan provides the most useful information, as opposed to the map. “I do think it’s important to look at the map, but a map is only a map because of segmentation lines that usually a computer creates of the different layers,” she says. “If there’s any kind of decreased signal strength or an artifact, that segmentation can be off and you can’t trust it, impacting things such as calculation of thickness in various quadrants. Those are all helpful only if the segmentation is accurate, so I look at those segments secondarily.”
As OCT devices evolve, Dr. Waheed says surgeons are seeing more. “Not only can we characterize disease better, but it can also be a prognostic tool,” she says. “As we get higher resolution and faster speeds, we can better visualize the retina overall and see things that may be harder to detect when you just look at the traditional cross-sectional images.”
But even as technology takes us deeper into the retina, surgeons have to be aware of what they’re looking at, says Dr. Grewal, and whether it represents true anatomy or pathology versus a potential artifact.
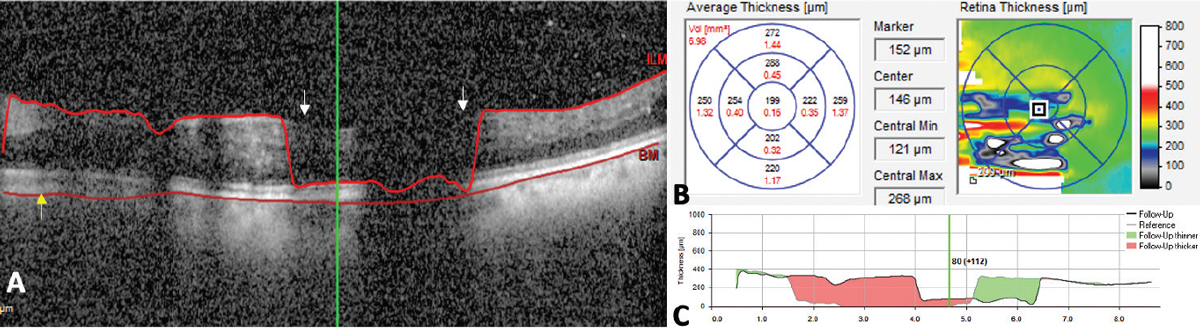 |
| Figure 2. Error in segmentation (A) due to failure of the algorithm to correctly identify the internal limiting membrane (white arrows) and Bruch’s membrane (yellow arrow). The resulting thickness maps that are generated are erroneous (B) and thickness change maps in such situations (C) compared to the prior scans aren’t accurate reflections of change in retinal thickness and shouldn’t be used to make treatment decisions. Photo: Dilraj Grewal, MD. |
“It comes down to how we use OCT in the assessment of patients. We use it both qualitatively and quantitatively,” he says. “For qualitative assessment, we can visualize different layers of the retina and vitreous down to the choroid and visualize the normal structure or variations of abnormalities on it. So, if there are artifacts in the image, that’s going to impact our qualitative assessment. For example, the image may appear to be very grainy, with a lot of white dots in the vitreous. One might interpret that as inflammation or the presence of blood in the vitreous, whereas the reality is that it may just be an artifact due to poor scan quality.”
Identifying and Rectifying Artifacts
Artifacts can be caused by several factors, including the patient, the device or software and the operator/imager.
Motion artifact is one of the most common issues caused by the patient, says Dr. Waheed. “If the acquisition is long, patients can move and you get a lot of artifacts because of motion,” she says. These artifacts can result in segmentation errors and potentially appear as abnormalities in the retinal nerve fiber layer thickness measurement.1
Unless recognized, this artifact could severely alter your treatment plan, says Dr. Grewal. “If there’s a motion artifact, then you may get irregular variations in the anatomy, which is again, an artifact causing a qualitative error of assessment rather than true pathology,” he says. “And in terms of quantification, any issue with scan quality may affect the segmentation of the retinal layers by the software.
“Measuring the thickness of different layers depends on accurate segmentation of the boundaries, so if these boundaries are erroneously depicted due to quality issues, then your quantitative measurements are going to be erroneous as well,” Dr. Grewal continues. “This has significant implications because you may, for example, be monitoring central subfield thickness (the thickness of the retina in the central one millimeter circle). If you’re using that to make treatment decisions, scan quality issues or artifacts may cause that value to be erroneously high or low, and that would impact your treatment decision. So, it’s very important to pay attention to the quality of the image prior to interpreting it whether qualitatively or quantitatively.”
Motion artifacts may be due to the patient’s inability to fixate, which depends on their vision, Dr. Grewal notes. “If they’re not able to fixate on the scan pattern—because any image that’s acquired is based on a particular scan pattern that the machine projects, whether it’s a raster, radial or circle scan—if the patient can’t track and follow during the scan, then the scan may be off the foveal center and there’s potential for the scan to have significant motion artifact,” he says.
Motion artifact can be prevented with clear instructions, a fixation pointer and artificial tears, advises Dr. Waheed.
Fortunately, evolving technology is contributing to reducing motion artifacts. “As scan acquisition time goes down, blinking (motion) doesn’t matter as much because you can get more of a scan before the patient feels they have to blink or move,” says Dr. Olmos de Koo.
The eye’s condition will also contribute to scan quality issues, says Dr. Grewal. “These issues may start all the way from the corneal tear film,” he says. “If the cornea is very dry, or there’s corneal edema, then it’s going to impact the penetration and reflectance of the light beam, which is what OCT is based on. This will hold true for any structure as you go inward.”
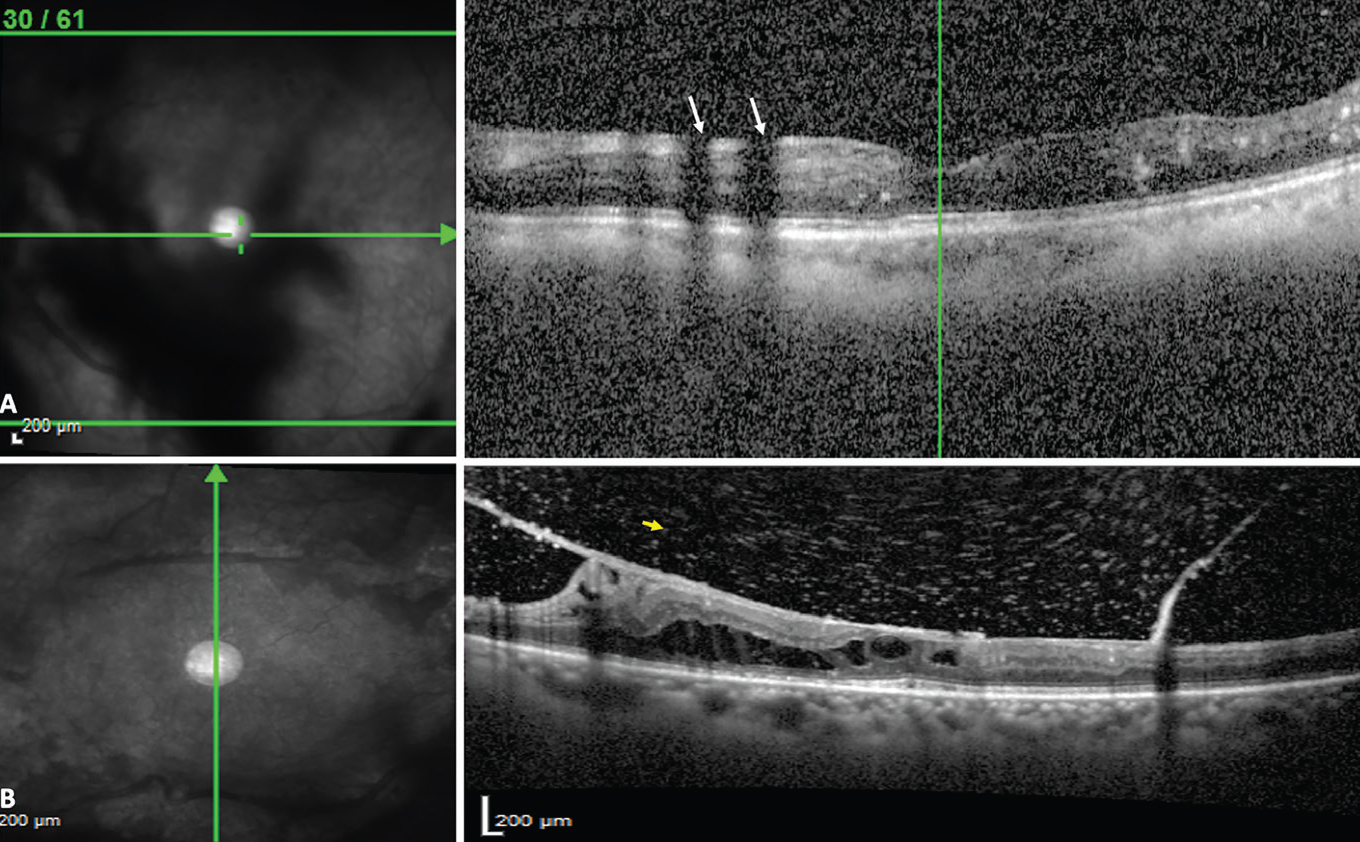 |
| Figure 3. Vitreous opacities causing a shadow artifact (A, white arrows). The white dots seen in the vitreous are due to poor image quality and aren’t true vitreous hyperreflective dots, which are seen in B, in a good quality OCT scan (yellow arrow). Photo: Dilraj Grewal, MD. |
“Rectifying a poor tear film or dry corneal surface is among the simplest issues to resolve,” Dr. Grewal continues. “Instilling artificial tears and asking the patient to blink frequently, as well as performing OCT early in the evaluation process as the patient gets worked up, are some of the strategies that can be used to mitigate the effects of poor scan quality due to the tear film.”
Artifacts are also connected to the devices themselves. “There are some issues inherent to the device and the software that lead to limitations of the technology,” notes Dr. Grewal. “These may include the field of view or the ability to visualize the peripheral retina, and issues with the software segmentation algorithm, where there may be failure of segmentation due to the software’s inability to detect the boundaries in the presence of significantly distorted anatomy.”
Misidentification of the retinal layers carries certain implications, with outer retinal layer misidentification being more significant. Srinivas R. Sadda, MD, of the Doheny Eye Institute in Los Angeles, reported errors in thickness measurement and retinal boundary detection in 92 percent of eyes2 undergoing Stratus OCT imaging. This was most severe in only 13.5 percent of eyes; it was attributed to a higher FCTSD-to-FCT ratio, as well as the presence of subretinal fluid.
Segmentation errors have been a significant issue, although they’re getting less problematic because the software works really well, says Dr. Waheed. “For instance, if you have outer retina line misidentification, that line is used to quantify how thick the retina is. And it’s important in quantifying, for example, if there’s fluid there and you’re using the quantification to make decisions about therapy. So if you have outer retinal misidentification—and this often happens in patients who have outer retinal disease like macular degeneration—then the quantification is inaccurate. Retina thickness is really important in a patient with macular degeneration or diabetic macular edema.”
This demonstrates the role pathology plays in artifacts. Dr. Olmos de Koo hesitates to connect any particular artifact to any machine, but says she wouldn’t be surprised if different depths of penetration lead to different artifacts. “There’s extended depth imaging, EDI, and there’s standard imaging. You might be more interested in the vitreoretinal interface, in which case you wouldn’t want to do EDI,” she says. “But when you need to focus on the choroid, EDI can give you a clearer picture of what’s below the retina and how it may impact retinal disease. In certain cases you can’t have it all and it’s best to focus your acquisition of images on the specific pathology that you’re looking at.”
Segmentation artifacts can be subtle, says Dr. Grewal. “Segmentation can be inaccurate either because of a focal scan quality like the shadow artifact from vitreous opacity, or because of focal areas of poor signal strength that could cause dropout of signal and therefore poorly resolved delineation of the retinal boundaries,” he says. “It’s often very helpful to look at the thickness maps to see if there are focal areas where there are irregularities, because you would typically expect the thickness map to be relatively smooth, following normal retinal anatomy with a foveal dip. If you’re seeing large variations, such as an inordinate variations in thickness in one area that don’t correlate with what you’re seeing on your exam, that should prompt you to determine why the thickness is affected, particularly if you’re using those values to make treatment decisions. That includes assessing whether you know there’s an epiretinal membrane or vitreomacular traction, or the layers haven’t been correctly segmented.
“The posterior hyaloid or the posterior boundary of the vitreous are hyper-reflective, the machine algorithm may recognize that as an internal limiting membrane, and therefore the thickness of the retina is going to be artificially increased,” Dr. Grewal continues. “When a patient presents with a very long eye, you may get some images where the scans may be flipped over or clipped. This is because the way OCT works is that there’s a certain scan width within which the OCT signal is going to be of the highest quality. If the eye is very long with a posterior staphyloma, then the scan may be flipped outside these limits and that’ll cause an artifact.”
In neovascular conditions such as diabetic retinopathy or macular degeneration, the presence of hemorrhage may cause scan quality issues. Dr. Grewal says, “If there’s a large submacular bleed in macular degeneration, then the penetration into the retina will be impacted due to the hyperreflective blood. If there’s a bleed in diabetic retinopathy, then your signal strength into the retina is going to be impacted.
“Some other factors that may cause such an impact include the presence of significant inflammation or blood in the anterior chamber, posterior synechiae in the iris, cataract and vitreous haze, blood or opacities in the vitreous cavity,” Dr. Grewal continues. “Some strategies may help to overcome the vitreous opacities and vitreous floaters for example—you can ask the patient to look around just prior to image acquisition—but that is again contingent on the opacities not being very dense.”
Another artifact found only in SD- OCT is the mirror artifact. “SD-OCT machines generate two OCT images which are symmetric around the zero-delay line,” Dr. Waheed explains. “If the retinal image crosses the zero-delay line anteriorly, the corresponding symmetric mirror image on the truncated side will cross into the scanning range and be seen as a ‘ghost’ mirror image. This artifact can be corrected by properly positioning the retina to avoid crossing the zero-delay line.”
Mirror artifacts happen frequently in myopic eyes, adds Dr. Olmos de Koo. “When you have a very highly curved eye, let’s say you have a myopic contour, and it’s very U-shaped, it can actually flip and make the retina look like it’s upside down,” she says. “This happens frequently in very myopic eyes, making those harder to image. When that happens, it’s very clear that it’s an artifact—you’re not going to mistake it for another disease. But those OCTs are harder to evaluate.”
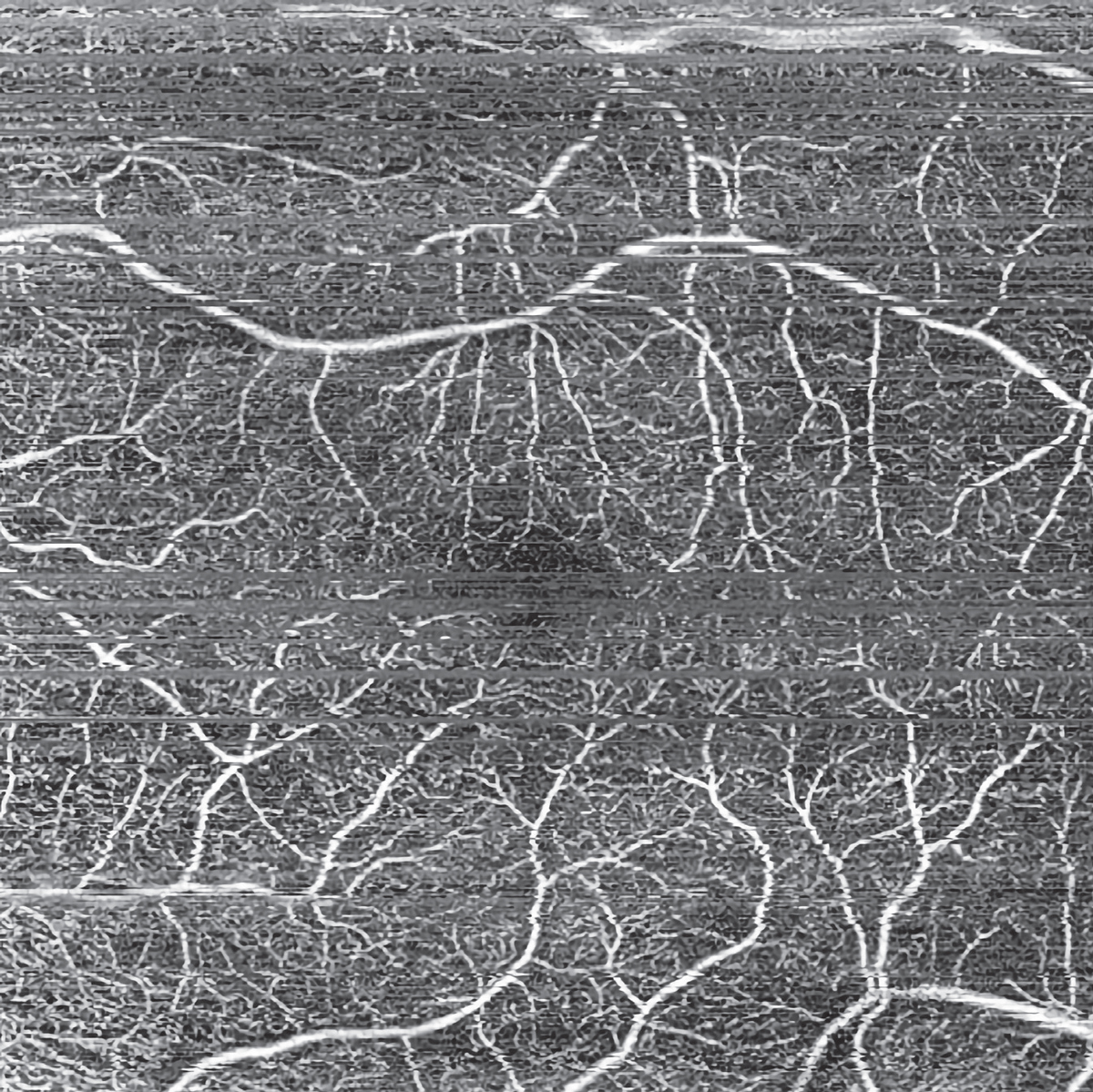 |
| Figure 4. An example of motion artifact (horizontal lines mainly seen in the upper third and center of the image) on a patient with diabetic retinopathy. Photo: Nadia Waheed, MD. |
Dr. Waheed says mirror artifacts can also occur in cases of massive retinal thickening, poor scan placement or elevation of retina due to RD, schisis or tumor.
Dr. Olmos de Koo says acquiring OCTs from challenging patients requires a lot of skill. “Experienced photographers are gems,” she says. To ensure her photographers obtain the best scan possible, she offers these tips: “I try to make sure the scans are centered on the fovea and capture the foveal center, and that they register to the prior scan for comparison,” she explains. “The person doing the scan also needs to make sure that the ocular surface is well-lubricated, so I keep a supply of artificial tears next to the machine. If there’s any concern at all, I encourage them to apply tears and reacquire the scan.”
Dr. Grewal agrees that technicians play a part in mitigating some factors. “In follow-up visits, it’s important to remember to register the follow-up images to the baseline image so that measurements are accurately compared, and to ensure that the same area of the retina is being scanned,” he says. “This is particularly important when the anatomy is distorted. If your normal foveal contour is lost in the presence of significant edema or scar tissue on the retina, and you’re not scanning the exact same area over time, then you may again get erroneous comparisons to baseline.”

|
Finally, the retina specialist must also take an active role in OCT accuracy. Dr. Grewal relies on a mental checklist. “It’s very helpful to have a checklist in terms of scan quality, looking for motion artifact, registration to the baseline scan, shadow artifact and whether the images are clipped,” he advises. “Also, have a defined scan protocol for your patients so that the potential variations are reduced as you go through your workflow.”
Often, doctors need to accept that some artifacts are simply unavoidable, explains Dr. Olmos de Koo. “You can get a lot of information from an ocular OCT. You just need to optimize your image acquisition and recognize the limitation of any artifacts, remembering that you can still get useful information even when there are artifacts,” she says. “With blink artifact or motion artifact, that’ll just give you poor signal strength or cause you to be unable to acquire a cube. Sometimes you only get a line scan—but you can get better resolution with just a line scan, rather than doing a whole cube or raster scan. And if that’s all you can get because of motion or blink or poor media, then you take that and go with it. You shouldn’t just discount the whole scan because there are some artifacts.”
Dr. Grewal reports no relevant disclosures. Dr. Olmos de Koo is a consultant for Alcon. Dr. Waheed is a consultant for Nidek and Topcon and her institution receives research support from Zeiss and Heidelberg.
1. Chhablani J, Krishnan T, Sethi V, Kozak I. Artifacts in optical coherence tomography. Saudi J Ophthalmol 2014;28:2:81-7.
2. Sadda SR, Wu Z, Walsh AC, Richine L, Dougall J, Cortez R, LaBree LD. Errors in retinal thickness measurements obtained by optical coherence tomography. Ophthalmology 2006;113:2:285-93.



