Autoimmune retinopathy (AIR) is a group of autoimmune degenerative retinal diseases characterized by stereotypical clinical, visual field, electrophysiologic and ocular imaging findings along with the presence of the circulating antiretinal antibodies (ARA).1 Though AIR is rare, that makes it more important to be aware of the signs, symptoms and imaging findings associated with it, in order to make an accurate diagnosis when and if it presents. In this article, we’ll review the epidemiology, diagnosis and treatment of AIR.
Epidemiology
AIR is thought to be a rare disease, constituting less than 1 percent of uveitis cases in one tertiary eye clinic.2 AIR can be divided into two groups, paraneoplastic and non-paraneoplastic. Paraneoplastic AIR includes cancer-associated retinopathy (CAR) and melanoma-associated retinopathy (MAR). Non-paraneoplastic AIR (npAIR) isn’t associated with an underlying malignancy and is a diagnosis of exclusion.
Non-paraneoplastic AIR is more common than paraneoplastic AIR.3 CAR is more prevalent than MAR.3 The average age of symptom onset ranges from 55 to 65 years, with npAIR skewed towards younger patients compared to paraneoplastic AIR.4–6 In patients with npAIR, comorbid autoimmune diseases are common, with a predilection for females.4,7 The diagnosis of cancer typically predates the symptoms associated with CAR and MAR, but the timeframe is variable.8 Rarely, ocular symptoms can precede the cancer diagnosis.9 Therefore, it’s necessary for the clinician to always remain suspicious. Numerous cancers have been associated with CAR, most commonly small-cell lung cancer.10 Other cancers which have been reported to be associated with CAR include:
- breast;
- ovarian;
- endometrial;
- cervical;
- non-small cell lung cancer;
- lymphoma;
- colon;
- pancreatic;
- prostate;
- bladder; and
- laryngeal cancers.10
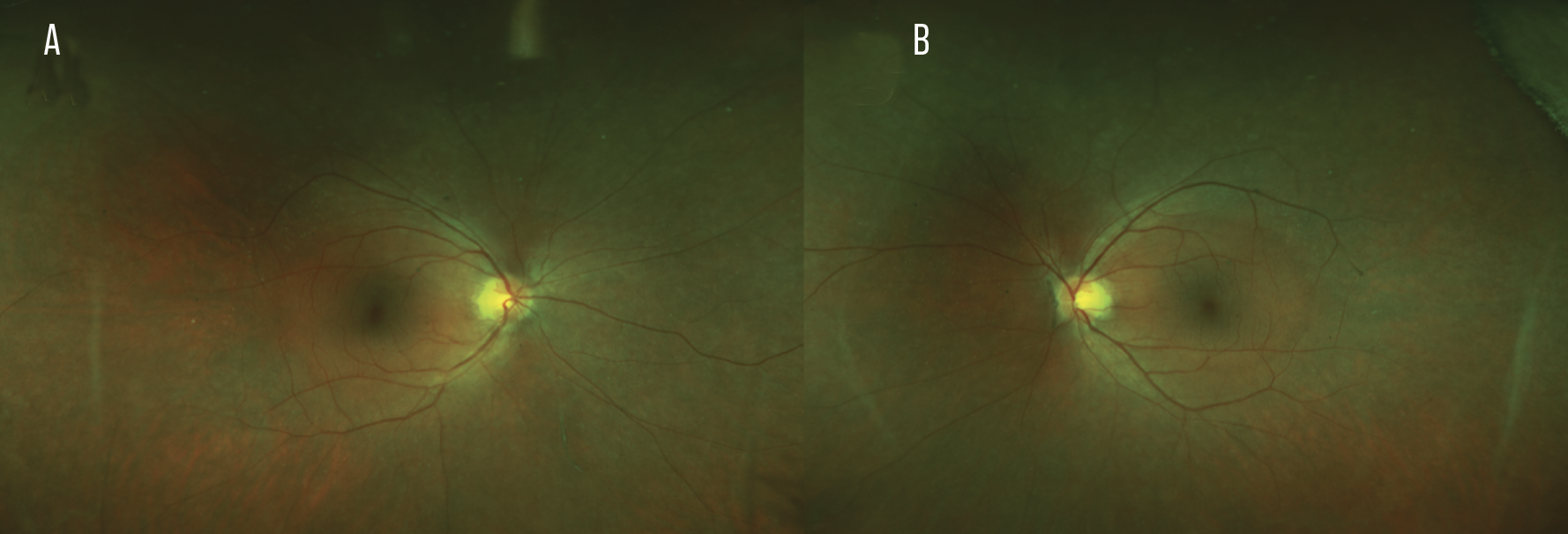 |
| Figure 1. Ultra-widefield fundus photos of an autoimmune retinopathy patient’s right (A) and left eyes (B) revealing diffuse bilateral but asymmetric retinal pigmented epithelial changes that spare the posterior pole along with arteriolar narrowing. |
Pathophysiology
The pathophysiology of AIR is largely presumptive and is based upon the presence of circulating ARA, which are thought to target retinal antigens resulting in disease manifestation. In the case of paraneoplastic AIR, molecular mimicry is the proposed pathogenic mechanism behind the disease, wherein antibodies formed against tumor antigens are thought to cross-react retinal antigens, resulting in disease.2 It’s been postulated that ARA are cytotoxic, inducing cell death after internalization through caspase-dependent apoptosis.11
Numerous ARA have been identified in the literature.1 The most commonly targeted protein of ARA in CAR is recoverin, a 23 kDa protein found in retinal photoreceptor cells.4 Another well described target of ARA is enolase, a 48kDa enzyme found in ganglion cells, Müller cells, rods and cones.4 Notably, AIR associated with anti-recoverin antibodies is associated with worse outcomes compared to AIR associated with anti-enolase antibodies.4
Curiously, the presence of serum ARA has been detected in 42 percent of normal healthy controls.12 ARA have also been detected in patients with various systemic autoimmune conditions, including Behçet’s disease, systemic lupus erythematosus, inflammatory bowel disease and multiple sclerosis, all without ocular complications.13–16 These findings suggest that the presence of ARA alone isn’t pathogenic. Finally, ARA have also been detected in 10 to 51 percent of patients with retinitis pigmentosa.17–19 RP and AIR present with similar clinical features, making clinical differentiation difficult. The high prevalence of ARA in patients with RP further blurs their differentiation. Interestingly, it’s been hypothesized that RP cases with prominent intraocular inflammation may be part of an overlap syndrome between the two diseases,19 especially when cystoid macular edema is present.20
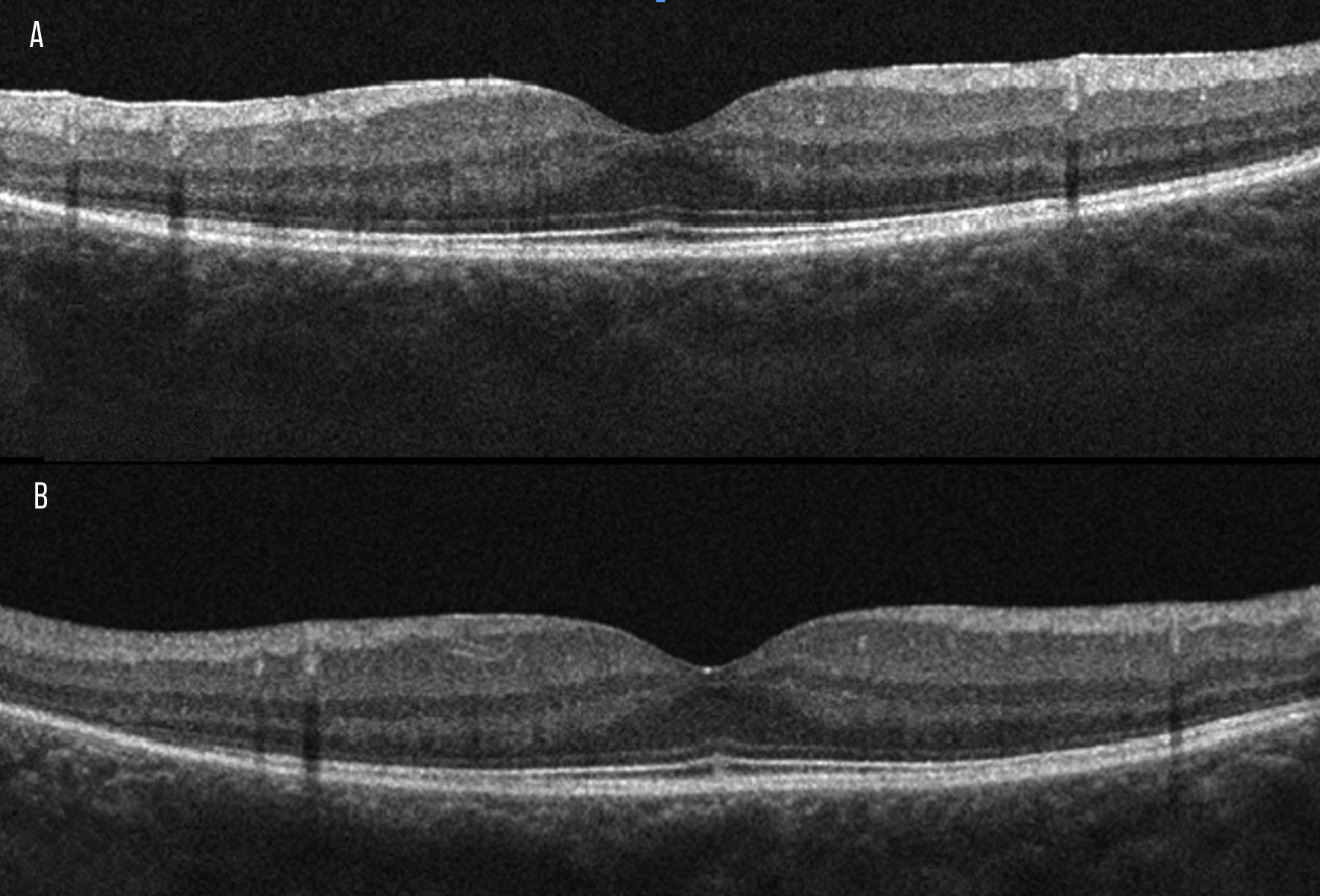 |
| Figure 2. Optical coherence tomography of an autoimmune retinopathy patient’s right (A) and left eyes (B) revealing peripheral loss of the outer retinal layers including the interdigitation zone, ellipsoid zone and external limiting membrane. |
Clinical Presentation and Diagnostic Imaging
The clinical findings of AIR are heterogenous and diverse.1 Patients with AIR most commonly present complaining of subacute vision loss, peripheral visual field loss, flashing lights and/or night blindness.2 Clinical examination in early disease may be unremarkable with a notable lack of intraocular inflammation.2,7,10 This absence of clinical findings not only makes the diagnosis of AIR difficult but may also lead to a delay in diagnosis and treatment. As the disease progresses, advanced findings include narrowing of the retinal vasculature, retinal pigmented epithelial abnormalities, optic nerve pallor and mild vitreous cells (Figure 1).2 AIR is usually bilateral but can be asymmetric.2 Visual acuity is usually preserved until late disease.2
A constellation of diagnostic imaging findings can increase suspicion for a diagnosis of AIR, and in conjunction with clinical exam and positive ARA are key to making a diagnosis of AIR.
Spectral domain optical coherence tomography may be the most useful ancillary imaging test in the context of AIR. Multiple studies have shown that AIR is associated with progressive outer retinal loss, particularly of the ellipsoid zone that begins peripherally and initially spares the fovea (Figure 2).21 Findings of outer retinal loss or EZ disruption may precede electroretinogram findings or ARA detection.22–25 Initial treatments of AIR didn’t appear to be associated with recovery of the EZ.21,23–25 However, a recent report by our team has shown significant recovery of the EZ when npAIR with cystoid macular edema is treated with anti-interleukin 6 medications (tocilizumab or sarilumab).26 Finally, OCT should be used to assess for cystoid macular edema, which is present in 24 to 66 percent of eyes17,21 and is a biomarker of more severe and more progressive disease.27
Depending on the stage of disease, fundus autofluorescence (FAF) can reveal a diffuse or stippled hyper-autofluorescent pattern throughout the posterior pole that initially spares the central macula. A parafoveal ring of abnormal hyperautofluorecence located between an area of normal autofluorescence inside the ring and hypoautoflourescence outside the ring has been reported as a characteristic finding in one small cohort with AIR (Figure 3).24 It’s been hypothesized that the hyperautofluorescent ring likely represents an abnormal collection of lipofuscin in the RPE due to enhanced outer segment turnover during apoptosis.28 Using FAF to monitor disease progression and response to treatment has been suggested.24
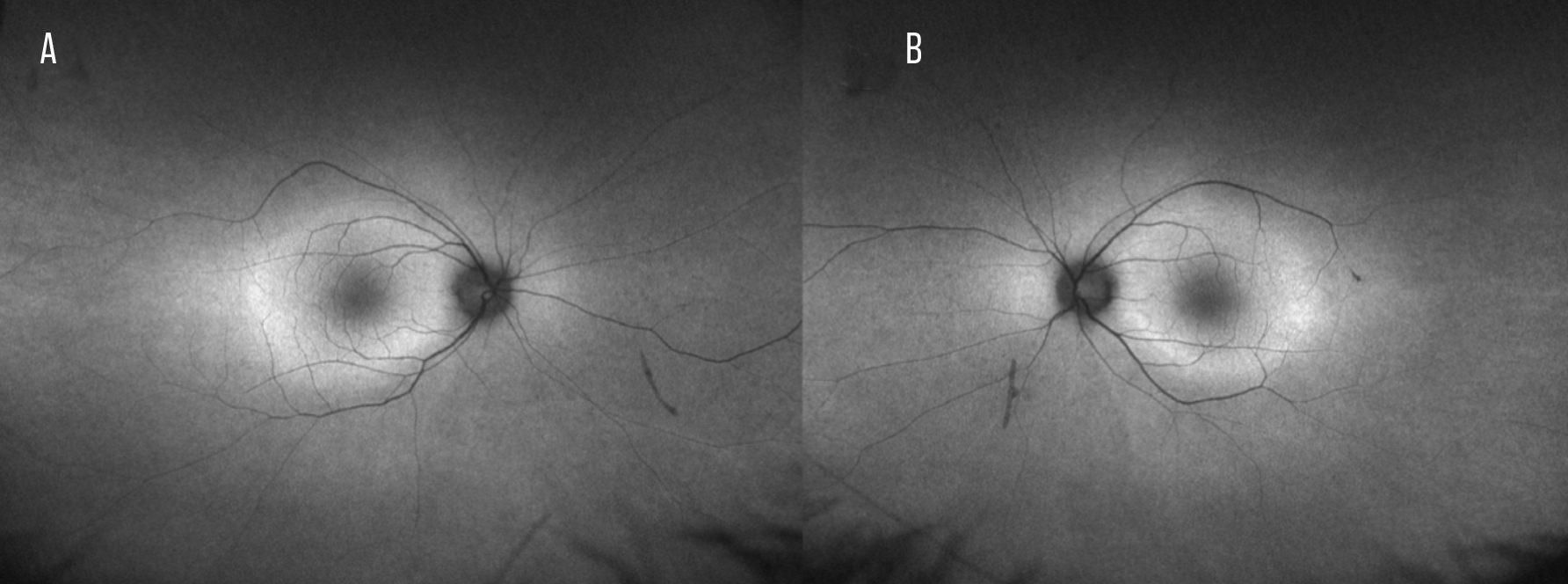 |
| Figure 3. Ultra-widefield fundus autofluorescence of an autoimmune retinopathy patient’s right (A) and left eyes (B) showing a ring of relative hyperautofluorescence in the macula surrounded by hypoautofluorescence in the periphery. |
Visual field testing typically reveals peripheral constriction (Figure 4).2 However, central or paracentral scotomas are also possible.2 Fluorescein angiography rarely shows leakage, but should be performed to rule out other possible etiologies.
There are no ERG findings that are diagnostic for AIR. ERG abnormalities have been reported in both full-field (ffERG) and multifocal electroretinogram (mfERG) including abnormal cone, rod and bipolar cell responses.29 Full-field ERGs may be more sensitive in detecting abnormalities in patients with AIR compared to multifocal ERGs.21
ERG can be particularly useful in some presentations of AIR, particularly those with CAR, MAR or anti-enolase antibodies. CAR is most commonly associated with anti-recoverin antibodies and ERG typically shows abnormalities in the cone responses,4,30 whereas MAR typically shows an electronegative waveform on ffERG.6 Finally, AIR associated with anti-enolase antibodies have been reported to have normal or near-normal ffERGs but very abnormal mfERGs.1
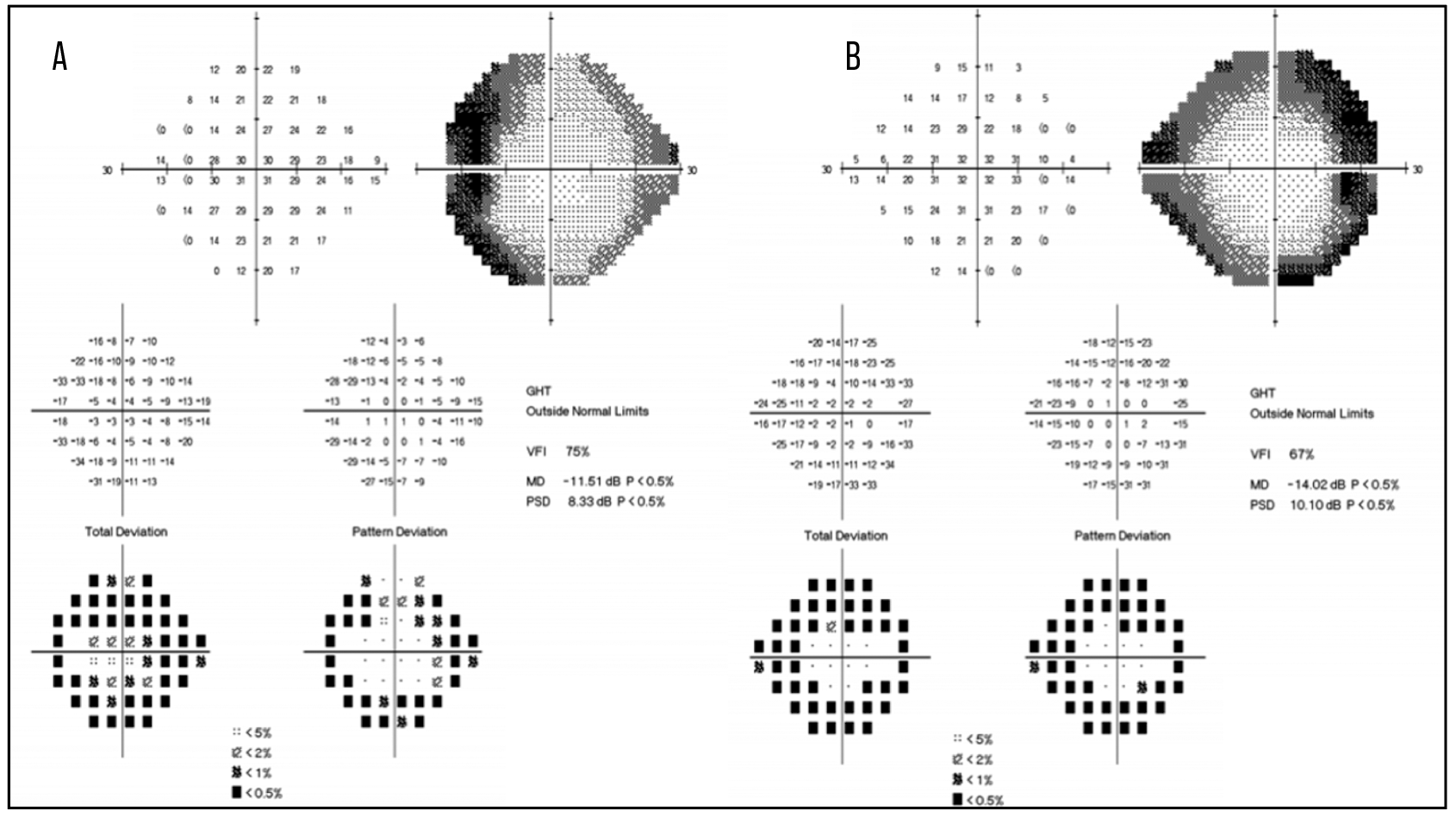 |
| Figure 4. Automated static perimetry of an autoimmune retinopathy patient’s right (A) and left eyes (B) revealing peripheral visual field constriction corresponding to her fundus photographs, optical coherence tomography scans and fundus autofluorescence in Figures 1-3. |
Work-up
As discussed previously, the presence of ARA isn’t diagnostic for AIR as they are present in a significant portion of the normal population.12 Rather, the presence of ARA in the setting of compatible clinical and diagnostic imaging is necessary for a diagnosis of AIR. The detection of ARA can be performed using a variety of laboratory techniques including immunohistochemistry (IHC), Western blot and enzyme-linked immunosorbent assay (ELISA).1 In brief, IHC involves exposing normal donor retina tissues to the suspect patient’s serum.1 If ARA are present, they bind to the retinal antigens and then are counterstained with a fluorescent-tagged anti-IgG, allowing for identification using confocal laser microscopy.1 Identification of ARA with Western blot involves separation of normal donor retina proteins by molecular weight using electrophoresis.1 These separated proteins are then exposed to the suspect patient’s serum.1 Again, if ARA are present, they bind to the retinal antigens and are then counterstained for IgG for identification.1 Finally, ELISA is a very sensitive laboratory technique which can be used to quantify the amount of ARA present using a colorimetric reaction and a spectrophotometer.1 A majority of patients with AIR have serum ARA against more than one retinal antigen.17
Systemic evaluation to rule out cancer is imperative in any patient with clinical or imaging concerns for AIR. Computed tomography of the chest, abdomen and pelvis along with magnetic resonance imaging of the brain should be performed to survey for any evidence of malignancy. Further investigation can be co-managed with the patient’s primary care provider and should include a thorough review of systems, complete physical examination, basic laboratory work-up, along with age and gender appropriate screening for colon, breast, gynecologic and prostate cancer. A full body skin check should be performed to assess for dermatologic malignancies, particularly melanoma.
The differential diagnosis consists of the following:
- retinitis pigmentosa;
- cone-rod dystrophy;
- toxic retinopathy;
- nutritional retinopathy;
- acute zonal outer occult retinopathy; and
- non-infectious and infectious uveitis syndromes.
The typical autoimmune retinopathy patient is a female in her mid 50’s or 60’s with no family history of retinitis pigmentosa or other inherited retinal dystrophies who presents with new onset flashes and peripheral visual field loss. If these symptoms are new in onset, funduscopic examination would likely appear normal. There should be limited, if any, intraocular inflammation. Review of possible toxic or nutritional deficits should be explored. If diagnostic imaging with OCT, FAF, ERG and visual field testing is consistent with findings reported in AIR, then you should perform ARA testing. If ARA are present, then be sure to perform a systemic evaluation for malignancy. If no malignancy is found, then you can assume a tentative diagnosis of npAIR.
Treatment
The management of AIR remains a challenge. High suspicion for a diagnosis of AIR allows for early diagnosis and treatment as therapy after widespread retinal degeneration occurs isn’t helpful.7,17 However, there is a lack of consensus in local or systemic treatment protocols for autoimmune retinopathy.31
If a diagnosis of CAR or MAR is made it is essential to eliminate or reduce the tumor burden through surgery, chemotherapy or radiotherapy, as indicated.6,32,33 Decreasing the tumor burden is believed to decrease the amount of ARA production.1
Systemic immunosuppressive therapy is often administered given the suspected pathophysiology. Various treatments have been described in retrospective case reports and case series, including:
- local and systemic corticosteroids;
- cyclosporine;
- mycophenolate;
- azathioprine;
- rituximab;
- ipilimumab;
- sarilumab;
- tocilizumab;
- intravenous immunoglobulin; and
- plasmapheresis.31
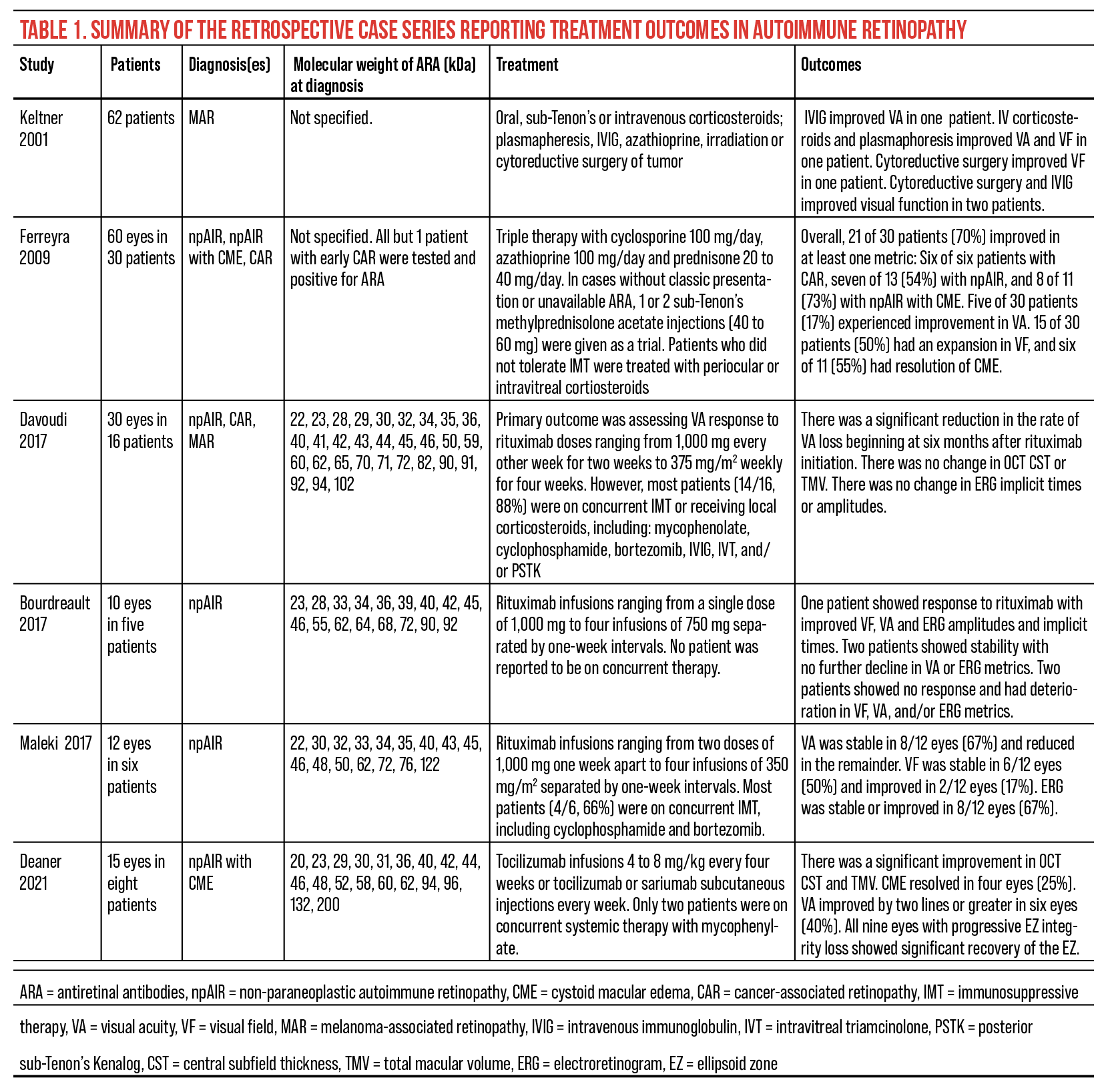
Given the heterogenous nature of this ambiguous disease, perhaps it’s unsurprising that the response to these treatments can be quite variable with most only slowing the progression of disease. However, we recently reported our success in treating npAIR with CME using the anti-interleukin 6 agents tocilizumab and sarilumab.26 Not only did we see significant improvement in OCT central subfoveal thickness and total macular volume metrics with resolution of CME in 25 percent of eyes, but there was also a trend towards improved visual acuity, with 40 percent of eyes improving by two lines or greater.26 Table 1 is a summary of the retrospective case series reporting treatment outcomes in autoimmune retinopathy.6,7,26,34–36
 |
| Figure 3. Ultra-widefield fundus autofluorescence of an autoimmune retinopathy patient’s right (A) and left eyes (B) showing a ring of relative hyperautofluorescence in the macula surrounded by hypoautofluorescence in the periphery. Click image to enlarge. |
Patients are typically followed with serial functional and anatomic testing with repeat OCT and FAF at every visit and visual fields and ERGs every three to six months with the goal of stabilization or recovery. Several case reports have described a decrease in circulating ARA following treatment and have advocated quantification of ARA as a possible method of assessing response to treatment.37–41 However, a recently published case series revealed no correlation between the quantitative change in ARA and clinical activity, suggesting that quantitative measurement of ARA shouldn’t be used in making management decisions.42
In conclusion, autoimmune retinopathy remains both a diagnostic and a management challenge. The ophthalmologist must remain alert and suspicious for this occult and heterogenous disease. Multimodal imaging may help to identify subtle signs, and in the setting of positive ARA make a diagnosis of AIR. Malignancy must be ruled out prior to initiating local or systemic immunosuppressive therapy. Both the patient and physician must be aware of the uncertain disease prognosis and that response to therapy is variable.
Dr. Bowe is a PGY-3 Ophthalmology Resident at Wills Eye Hospital.
Dr. Deaner is an assistant professor of ophthalmology at the Sidney Kimmel Medical College at Thomas Jefferson University. He practices at Mid Atlantic Retina, Wills Eye Hospital. He can be reached at jdeaner@midatlanticretina.com.
Dr. Regillo is the director of the Retina Service of Wills Eye Hospital, a professor of ophthalmology at Thomas Jefferson University School of Medicine and the principle investigator for numerous major international clinical trials.
Dr. Yonekawa is an assistant professor of ophthalmology at Sidney Kimmel Medical College at Thomas Jefferson University. He serves on the Education Committee of the American Society of Retina Specialists and on the Executive Committee for the Vit Buckle Society, where he is also the vice president for academic programming.
1. Grewal DS, Fishman GA, Jampol LM. Autoimmune retinopathy and antiretinal antibodies: A review. Retina Phila Pa 2014;34:5:827-845.
2. Grange L, Dalal M, Nussenblatt RB, Sen HN. Autoimmune retinopathy. Am J Ophthalmol 2014;157:2:266-272.
3. Canamary AM, Takahashi WY, Sallum JMF. Autoimmune retinopathy: A review. Int J Retina Vitr 2018;4:1.
4. Adamus G, Ren G, Weleber RG. Autoantibodies against retinal proteins in paraneoplastic and autoimmune retinopathy. BMC Ophthalmol 2004;4:5.
5. Adamus G. Autoantibody targets and their cancer relationship in the pathogenicity of paraneoplastic retinopathy. Autoimmun Rev 2009;8:5:410-414.
6. Keltner JL, Thirkill CE, Yip PT. Clinical and immunologic characteristics of melanoma-associated retinopathy syndrome: Eleven new cases and a review of 51 previously published cases. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc 2001;21:3:173-187.
7. Ferreyra HA, Jayasundera T, Khan NW, He S, Lu Y, Heckenlively JR. Management of autoimmune retinopathies with immunosuppression. Arch Ophthalmol Chic Ill 1960 2009;127:4:390-397.
8. Adamus G, Aptsiauri N, Guy J, Heckenlively J, Flannery J, Hargrave PA. The occurrence of serum autoantibodies against enolase in cancer-associated retinopathy. Clin Immunol Immunopathol 1996;78:2:120-129.
9. Saito W, Kase S, Ohguro H, Furudate N, Ohno S. Slowly progressive cancer-associated retinopathy. Arch Ophthalmol Chic Ill 1960 2007;125:10:1431-1433.
10. Chan JW. Paraneoplastic retinopathies and optic neuropathies. Surv Ophthalmol 2003;48:1:12-38.
11. Shiraga S, Adamus G. Mechanism of CAR syndrome: Anti-recoverin antibodies are the inducers of retinal cell apoptotic death via the caspase 9- and caspase 3-dependent pathway. J Neuroimmunol 2002;132:1-2:72-82.
12. Ko AC, Brinton JP, Mahajan VB, et al. Seroreactivity against aqueous-soluble and detergent-soluble retinal proteins in posterior uveitis. Arch Ophthalmol Chic Ill 1960 2011;129:4:415-420.
13. Rahi AH, Addison DJ. Autoimmunity and the outer retina. Trans Ophthalmol Soc U K 1983;103(Pt 4):428-437.
14. Forooghian F, Adamus G, Sproule M, Westall C, O’Connor P. Enolase autoantibodies and retinal function in multiple sclerosis patients. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol 2007;245:8:1077-1084.
15. Lee JH, Cho SB, Bang D, et al. Human anti-alpha-enolase antibody in sera from patients with Behçet’s disease and rheumatologic disorders. Clin Exp Rheumatol 2009;27(2 Suppl 53):S63-66.
16. Vermeulen N, Arijs I, Joossens S, et al. Anti-alpha-enolase antibodies in patients with inflammatory bowel disease. Clin Chem 2008;54:3:534-541.
17. Heckenlively JR, Ferreyra HA. Autoimmune retinopathy: A review and summary. Semin Immunopathol 2008;30:2:127-134.
18. Heckenlively JR, Aptsiauri N, Nusinowitz S, et al. Investigations of antiretinal antibodies in pigmentary retinopathy and other retinal degenerations. Trans Am Ophthalmol Soc 1996;94:179-200; discussion 200-206.
19. McMurtrey JJ, Tso MOM. A review of the immunologic findings observed in retinitis pigmentosa. Surv Ophthalmol 2018;63:6:769-781.
20. Heckenlively JR, Jordan BL, Aptsiauri N. Association of antiretinal antibodies and cystoid macular edema in patients with retinitis pigmentosa. Am J Ophthalmol 1999;127:5:565-573.
21. Khanna S, Martins A, Oakey Z, et al. Non-paraneoplastic autoimmune retinopathy: Multimodal testing characteristics of 13 cases. J Ophthalmic Inflamm Infect 2019;9:1:6.
22. Abazari A, Allam SS, Adamus G, Ghazi NG. Optical coherence tomography findings in autoimmune retinopathy. Am J Ophthalmol 2012;153:4:750-756, 756.e1.
23. Pepple KL, Cusick M, Jaffe GJ, Mruthyunjaya P. SD-OCT and autofluorescence characteristics of autoimmune retinopathy. Br J Ophthalmol 2013;97:2:139-144.
24. Lima LH, Greenberg JP, Greenstein VC, et al. Hyperautofluorescent ring in autoimmune retinopathy. Retina Phila Pa 2012;32:7:1385-1394.
25. Sepah YJ, Sadiq MA, Hassan M, et al. Assessment of retinal structural and functional characteristics in eyes with autoimmune retinopathy. Curr Mol Med 2015;15:6:578-586.
26. Deaner JD, Jaffe GJ, Keenan RT, Carnago L, Grewal DS. Anti-interleukin-6 antibodies for autoimmune retinopathy with macular edema. Ophthalmol Retina 2022;6:1:91-93.
27. Finn AP, Thomas AS, Stinnett SS, Keenan RT, Grewal DS, Jaffe GJ. The role of cystoid macular edema as a marker in the progression of non-paraneoplastic autoimmune retinopathy. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol 2018;256:10:1867-1873.
28. Lima LH, Greenberg JP, Greenstein VC, et al. Hyperautofluorescent ring in autoimmune retinopathy. Retina Phila Pa 2012;32:7:1385.
29. Weleber RG, Watzke RC, Shults WT, et al. Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with antienolase antibodies. Am J Ophthalmol 2005;139:5:780-794.
30. Whitcup SM, Vistica BP, Milam AH, Nussenblatt RB, Gery I. Recoverin-associated retinopathy: A clinically and immunologically distinctive disease. Am J Ophthalmol 1998;126:2:230-237.
31. Dutta Majumder P, Marchese A, Pichi F, Garg I, Agarwal A. An update on autoimmune retinopathy. Indian J Ophthalmol 2020;68:9:1829-1837.
32. Stead RE, Fox MA, Staples E, Lim CS. Delayed presentation of melanoma-associated retinopathy and subsequent resolution with cytoreduction surgery. Doc Ophthalmol Adv Ophthalmol 2013;127:2:165-171.
33. Powell SF, Dudek AZ. Treatment of melanoma-associated retinopathy. Curr Treat Options Neurol 2010;12:1:54-63.
34. Davoudi S, Ebrahimiadib N, Yasa C, et al. Outcomes in autoimmune retinopathy patients treated with rituximab. Am J Ophthalmol 2017;180:124-132.
35. Boudreault K, Justus S, Sengillo JD, et al. Efficacy of rituximab in non-paraneoplastic autoimmune retinopathy. Orphanet J Rare Dis 2017;12:1:129.
36. Maleki A, Lamba N, Ma L, Lee S, Schmidt A, Foster CS. Rituximab as a monotherapy or in combination therapy for the treatment of non-paraneoplastic autoimmune retinopathy. Clin Ophthalmol Auckl NZ 2017;11:377-385.
37. Eltabbakh GH, Hoogerland DL, Kay MC. Paraneoplastic retinopathy associated with uterine sarcoma. Gynecol Oncol 1995;58:1:120-123.
38. Guy J, Aptsiauri N. Treatment of paraneoplastic visual loss with intravenous immunoglobulin: Report of 3 cases. Arch Ophthalmol Chic Ill 1960 1999;117:4:471-477.
39. Keltner JL, Thirkill CE, Tyler NK, et al. Management and monitoring of cancer-associated retinopathy. Arch Ophthalmol Chic Ill 1960 1992;110:1:48-53.
40. Murphy MA, Thirkill CE, Hart WM. Paraneoplastic retinopathy: A novel autoantibody reaction associated with small-cell lung carcinoma. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc 1997;17:2:77-83.
41. Thirkill CE, FitzGerald P, Sergott RC, Roth AM, Tyler NK, Keltner JL. Cancer-associated retinopathy (CAR syndrome) with antibodies reacting with retinal, optic-nerve, and cancer cells. N Engl J Med 1989;321:23:1589-1594.
42. Stanwyck LK, Moussa K, Chan W, et al. Lack of correlation between number of antiretinal antibodies and clinical outcome measures in autoimmune retinopathy patients. Ophthalmol Retina 2019;3:11:1007-1009.




