Neurodegenerative diseases are the leading cause of disability around the world, and this burden is expected to increase exponentially over the next several decades.1 Alzheimer’s disease (AD), accounting for 60 to 80 percent of dementia cases, remains plagued by underdetection due to diagnostic barriers.2 Currently, brain tissue histopathology at autopsy remains the only definitive diagnosis of AD, and alternate diagnostic tools such as positron emission tomography scans and cerebrospinal fluid testing remain underutilized due to cost, invasiveness and lack of widespread availability.3,4 Identification of more widely applicable biomarkers to diagnose and monitor neurodegenerative diseases, especially preclinically when lifestyle changes may delay onset, remains an unmet need.
Retinal imaging provides a noninvasive and low-recurring-cost opportunity to capture structural changes to the neurosensory retina and its microvasculature, both of which may serve as biomarkers for cognitive impairment and neurodegenerative disease.5,6 Here, we describe the evolution and limitations of OCTA as a potential diagnostic tool for neurodegenerative disease, along with its utility as an input into machine-learning algorithms.
OCTA’s Potential
Optical coherence tomography angiography offers a 3-dimensional image of the retinal and choroidal structure and vasculature, allowing for the observation of vascular density and thickness of the different layers. Because OCTA essentially captures motion-contrast images, it may allow detection of retinal vessel density (VD) and perfusion density (PfD) at high resolution, and this microvasculature may demonstrate abnormalities earlier than larger retinal vessels visualized on retinal photographs.
Before exploring OCTA’s applications, it’s important to define the terms used for characterizing the microvasculature using OCTA: VD and PfD. Their structural definition varies to some extent with different OCTA platforms such as the Spectralis (Heidelberg), Solix (Visionix), Xephilio (Canon Medical Systems), and Angioscan (Nidek). On the Zeiss Cirrus HD-5000 Angioplex OCTA platform (Carl Zeiss Meditec), VD is defined as the total length of perfused vasculature per unit area within the region of measurement (mm/mm2). Perfusion density is defined as the total area of perfused vasculature per unit area in the region of measurement (percentage). For the 3x3-mm scans, VD and PfD are measured in the Early Treatment Diabetic Retinopathy Study (EDTRS) 3-mm circle and ring. For 6x6-mm scans, the VD and PfD are measured in the ETDRS 6-mm circle, inner ring and outer ring.
Our initial work at the Duke Eye Multimodal Imaging in Neurodegenerative Disease (iMIND) research study group reported a relationship between both reduced VD and enlarged foveal avascular zone (FAZ) area and AD using an OCTA-based comparative assessment.7 Early studies used OCTA to visualize VD in AD, as well as to find significant correlations between Mini Mental State Examination scores and FAZ area.8 Such findings have been the foundation to current literature highlighting OCTA as a potential clinical tool to identify biomarkers of AD.7,9 Of note, those carrying the apolipoprotein ε4 gene, a known risk factor for AD, showed variations in retinal layer thickness over time, highlighting potential early biomarkers of asymptomatic patients at higher risk of AD.10
 |
|
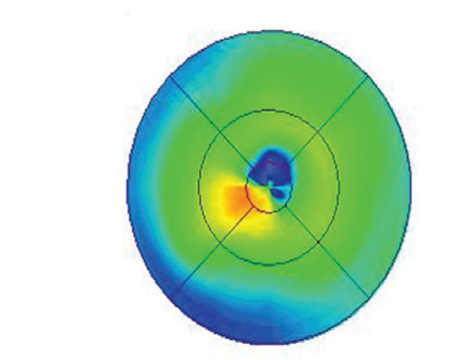
Figure 1. OCTA images showing differences in the retinal microvasculature among individuals with Alzheimer’s disease, Parkinson’s disease, mild cognitive impairment and age-matched controls. OCT images showing corresponding retinal nerve fiber layer (RNFL) and ganglion cell-inner plexiform layer (GC-IPL) thickness findings are included on the right. Adapted from Grewal et al. (2022).11 |
Mild cognitive impairment (MCI) is often a transitional state before the development of AD, making an early diagnosis imperative. Thus, OCTA has previously been used as a tool to identify significant microvascular loss among those with MCI.12 Among those with amnestic MCI, which is more likely to progress to clinical AD, PfD was significantly lower compared to non-amnestic MCI patients and controls.13
Several cross-sectional studies have also shown the utility of OCTA in identifying biomarkers of Parkinson’s disease.11,14,15 We previously demonstrated structural choroidal changes, as well as decreased VD and PfD, among those with PD characterized by OCTA (Figure 1). However, because PD has an average disease course of more than 14 years, the use of OCTA to study retinal alterations over time provides additional vital insights into the pathophysiology and progression of PD and how it may differ from normal age-related changes in OCTA and OCT metrics. Duke University’s Anita Kundu and co-workers used OCTA to examine longitudinal retinal structural and microvasculature changes among patients with PD and found a significantly faster rate of decline in VD, PfD and ganglion cell-inner plexiform layer (GC-IPL) thickness among those with PD, compared to the age-related decline seen in controls. PD patients with more advanced disease displayed faster rates of VD decline.16
In addition to AD and PD, OCTA imaging continues to show promise in biomarker identification for other neurodegenerative diseases including Traumatic Brain Injury (TBI) and Huntington’s Disease. Traumatic brain injury has been established as one of the strongest epigenetic risk factors for development of dementia. Among TBI patients, OCTA can be used to identify secondary structural changes in the retina in the absence of visual symptoms.17 Elahe Amini, MD, of the Iran University of Medical Sciences and her co-authors demonstrated reduced macular thickness and peripapillary retinal nerve fiber layer, and a paper authored by Duke’s Alice Haystead observed decreased VD, GC-IPL thickness and FAZ area among those with Huntington’s Disease.18,19 While such relationships should be confirmed with larger studies, it demonstrates the utility of OCT/OCTA across a spectrum of neurodegenerative diseases.
OCTA and AI as Diagnostic Tools
Machine learning models have recently been used to provide clinical diagnoses of AD and MCI with brain MRI and PET images.20 Retinal images may also be used as quantitative inputs to machine learning models, with lower costs and easier access. Jing Tian and co-workers at the Alzheimer’s Disease Research Center used publicly available UK Biobank data to train a modular machine learning model that accurately classified patients with AD 82 percent of the time.21
Recent work by the Duke iMIND Study Group has used OCTA images as an input into neural network models to determine the probability of neurodegenerative disease.22 A data set of 154 eyes from 80 patients with MCI were fed into the convolutional neural network (CNN) model, with 30 eyes used for testing (remaining 124 used for training and validation), and a probability score was produced, indicating likelihood that a patient would carry a clinical diagnosis of MCI. OCTA images were used as quantitative inputs to capture potential vascular features, while GC-IPL images were used in conjunction to capture potential structural features, yielding an 80.9 percent probability score. Using these combined inputs, the best performing model achieved sensitivity of 79 percent and specificity of 83 percent, performing comparably to a previous CNN differentiating control and AD patients using OCTA multimodal inputs.23 The combined CNN model yielded an area under the curve (AUC) of 0.836 while the model using only imaging inputs yielded an AUC of 0.829. Thus, the results suggest that the feasibility of creating a predictive risk model using only OCTA images as inputs. The model showed higher rates of specificity when given quantitative and demographical inputs. This suggests that a machine learning model may provide predictive capacity a significant amount of time prior to clinical symptom onset.
Congruence Between OCTA and Brain Imaging
Considering the proximity and shared embryological origin of the retina and brain, the use of OCTA images to reflect—and even predict—neurological changes would make sense; however, literature examining correlations between retinal and brain imaging is limited. Our group has demonstrated a negative correlation between lateral ventricle volume and OCTA VD, suggesting a relationship between OCTA imaging and cerebral biomarkers.24 Such findings are consistent with earlier associations between retinal thinning and medial temporal lobe atrophy.25 Other studies, although limited in sample size, have shown associations between OCTA parameters and fewer white matter hyperintensities (WMH), a precursor to cognitive decline and AD.26 These relationships between OCTA-identified biomarkers and neurological biomarkers in neurodegenerative disease, however warrant further investigation with longitudinal follow-up, and larger, more diverse and heterogenous populations.
Limitations of OCTA in Clinical Settings
Despite the growth of OCTA as a tool for identifying biomarkers in neurodegenerative diseases, there remain several unsolved challenges. First, characteristics measured by OCTA can be heterogenous across individuals, making it difficult to apply them broadly to diverse cohorts. As additional studies with larger and more diverse datasets become available, such limitations may be reduced. Longitudinal studies, such as that of Kundu et al., also provide a unique opportunity to examine relationships between OCTA and neurodegenerative disease over time and may confer a unique opportunity to assess the impact of disease-modifying therapies.16
The existence of confounding ocular diseases such as glaucoma, diabetic retinopathy, and age-related macular degeneration need to be accounted for when using OCTA in clinical settings. This is particularly relevant given the prevalence of glaucoma and AMD in the elderly population vulnerable to neurodegenerative diseases. The majority of neural network models to date have been built on otherwise healthy patients with no known confounding ocular or systemic diseases. Differentiating patterns of change between ocular diseases and preclinical and clinical neurodegenerative disease will be a key step to increase the generalizability of OCT- and OCTA-based retinal imaging models.
Another limitation is inaccurate information from OCTA analysis due to image quality issues and resulting artifacts. Good image quality is an essential prerequisite for accurate characterization of retinal and choroidal changes. For example, neurodegenerative disease patients with impaired motor function or head tremor may yield less reliable and repeatable OCTA scans. Towards this end, a paper from Duke’s Justin Ma demonstrated that the OCTA repeatability—measured by intraclass correlation coefficients—was good to excellent among those with AD, MCI and PD. The researchers also noted that interocular asymmetry in peripapillary OCTA wasn’t significant, indicating that single-eye imaging may present an option for future studies.27 Facilitating a shorter image acquisition time could minimize the limitation of motion artifacts. It’s critical to screen images carefully for quality control prior to using them for analysis. Our group recently created a neural network model that could help automate the time-consuming and resource-intensive task of assessing image quality for OCT and OCTA images. The model assessing OCTA image quality achieved an AUC of 0.83 while the combined model assessing both OCTA and GC-IPL images reached an AUC of 0.99.28 These results show that neural networks can be trained to sort through good-quality and poor-quality retinal images with accuracy, as well as integrate with existing automated model pipelines to help classify neurodegenerative diseases.
Also, it’s important to consider the varying OCTA platforms and scan types used. A previous comparison of five OCTA systems found poor agreement between systems, highlighting the variability that can occur when using OCTA across different imaging platforms.29 Differences in VD, PfD and FAZ aren’t cross-applicable across OCTA systems. Such barriers highlight the challenges of standardizing OCT, particularly OCTA images. One study found that while OCTA can identify biomarkers of AD, there remained significant heterogeneity across studies, largely attributable to different retinal layer segmentation algorithms and different definitions and thresholding algorithms for metrics like VD and PfD.30 Recommendations to standardize OCTA inputs across platforms have been put forward, and efforts to establish consensus among researchers, industry and regulators are critical to improve the clinical applicability across settings.
As the field of artificial intelligence flourishes, emerging machine learning architectures provide great promise in simplifying diagnostic efforts for neurodegenerative diseases. Improvements in our databases and AI technology would help overcome some of the limitations of current neural networks. These include techniques to reduce the risk of overfitting with small datasets, wherein the model associates images with certain outputs rather than learning why particular inputs are predictive of certain outputs. As the training population and different imaging platforms used for data collection grow, the generalizability to other populations and imaging platforms should also increase.
In conclusion, the role of OCT and OCTA-based retinal imaging in neurodegenerative diseases is rapidly expanding. A growing body of literature now shows the potential of OCTA detecting microvascular biomarkers for MCI, AD, PD and other neurodegenerative diseases, including TBI. While additional studies are needed to overcome the heterogeneity across OCTA platforms, we’ve made significant progress in evaluating OCT- and OCTA-based retinal biomarkers in the diagnosis and progression of neurodegenerative disease, and this is just the beginning. With advances in imaging technologies, analytics and data processing, OCTA is anticipated to have a greater role in the diagnosis and potential management of neurodegenerative diseases.
Joshua Woo is a first year medical student at the Duke University School of Medicine (DUSOM) in Durham. Sejal Patel is a second-year undergraduate student at Duke University in the Trinity College of Arts and Sciences studying Neuroscience. They both currently work with Duke’s iMIND (Eye Multimodal Imaging in Neurodegenerative Disease) Research Group.
Dr. Fekrat is a professor of ophthalmology, associate professor in the Department of Surgery and professor of neurology at Duke. She’s also director of the iMIND Research Group.
Dr. Grewal is an associate professor of ophthalmology and neurology at Duke.
None of the authors have a financial interest in the material presented.
Direct correspondence to Dr. Grewal at 2351 Erwin Road, Durham, NC 27710. dilraj.grewal@duke.edu. (919) 684-8111.
Dr. Regillo is the director of the Retina Service of Wills Eye Hospital, a professor of ophthalmology at Thomas Jefferson University School of Medicine and the principle investigator for numerous major international clinical trials.
Dr. Yonekawa is an assistant professor of ophthalmology at Sidney Kimmel Medical College at Thomas Jefferson University. He serves on the Education Committee of the American Society of Retina Specialists and on the Executive Committee for the Vit Buckle Society, where he is also the vice president for academic programming.
1. Feigin VL, Vos T, Nichols E, et al. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurology 2020;19:3:255-265.
2. Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia 2020;16:391–460.
3. Liao H, Zhu Z, Peng Y. Potential utility of retinal imaging for Alzheimer’s disease: A review. Frontiers in Aging Neuroscience 2018;10:188.
4. Kim JC, Goldman S, Racke MK, Clarke NJ. PS-136 – A new LC-MS/MS assay for the quantification of Ab40 and Ab42 in plasma: Validation and clinical performance. Presented at: Alzheimer’s Association International Conference. San Diego, 2022.
5. Chan VTT, Sun Z, Tang S, et al. Spectral-domain OCT measurements in Alzheimer’s disease: A systematic review and meta-analysis. Ophthalmology 2019;126:4:497-510.
6. Lad EM, Mukherjee D, Stinnett SS, et al. Evaluation of inner retinal layers as biomarkers in mild cognitive impairment to moderate Alzheimer’s disease. PLoS One 2018;13:2:e0192646.
7. Grewal DS, Polascik BW, Hoffmeyer GC, Fekrat S. Assessment of differences in retinal microvasculature using oct angiography in Alzheimer’s disease: A twin discordance report. Ophthalmic Surgery, Lasers and Imaging Retina 2018;49:6:440-444.
8. Bulut M, Kurtuluş F, Gözkaya O, et al. Evaluation of optical coherence tomography angiographic findings in Alzheimer’s type dementia. British Journal of Ophthalmology 2018;102:2:233-237.
9. Robbins CB, Grewal DS, Stinnett SS, et al. Assessing the retinal microvasculature in individuals with early and late-onset Alzheimer’s disease. Ophthalmic Surgery, Lasers and Imaging Retina 2021;52:6:336-344.
10. Ma JP, Robbins CB, Lee JM. Longitudinal analysis of the retina and choroid in cognitively normal individuals at higher genetic risk of Alzheimer’s disease. Ophthalmology Retina 2022;6:7:607-619.
11. Grewal DS, Sadda SR, Fekrat S. Understanding the role of the retina specialist in systemic diagnoses. Retina Times 2022;40:2:94.
12. Yoon SP, Grewal DS, Thompson AC, et al. Retinal microvascular and neurodegenerative changes in Alzheimer’s disease and mild cognitive impairment compared with control participants. Ophthalmology Retina 2019;3:6:489-499.
13. Grewal DS, Fekrat S. Assessment of retinal microvascular alterations in individuals with amnestic and non-amnestic mild cognitive impairment using optical coherence tomography angiography. RETINA 2022;42:1338-1346.
14. Robbins CB, Thompson AC, Bhullar PK, et al. Characterization of retinal microvascular and choroidal structural changes in Parkinson’s disease. JAMA Ophthalmology 2021;139:2:182-188.
15. Zou J, Liu K, Li F, et al. Combination of optical coherence tomography (OCT) and OCT angiography increases diagnostic efficacy of Parkinson’s disease. Quantitative Imaging in Medicine and Surgery 2020;10:10:1930-1939.
16. Kundu A, Ma JP, Robbins CB, et al. Longitudinal analysis of retinal microvascular and choroidal imaging parameters in Parkinson’s disease compared to controls. Ophthalmology Science 2023;100393.
17. Justin GA, Winslow L, Robbins CB, et al. Evaluating the retinal architecture and microvasculature in traumatic brain injury using optical coherence tomography angiography. Presented at The Retina Society. Pasadena, 2022.
18. Amini E, Moghaddasi M, Habibi SAH, et al. Huntington’s disease and neurovascular structure of retina. Neurological Sciences 2022;43:5933-5941.
19. Haystead A, Joseph S, Allen A, et al. Characterizing the retina structure and microvasculature in Huntington’s disease. ARVO, 2023.
20. Liu M, Cheng D, Wang K, et al. Multi-modality cascaded convolutional neural networks for Alzheimer’s disease diagnosis. Neuroinformatics 2018;16:3-4:295-308.
21. Tian J, Smith G, Guo H, et al. Modular machine learning for Alzheimer’s disease classification from retinal vasculature. Scientific Reports 2021;11:238.
22. Wisely CE, Richardson A, Henao R, et al. A convolutional neural network using multimodal retinal imaging for differentiation of mild cognitive impairment from normal cognition. Ophthalmology Science 2023;100355.
23. Wisely CE, Wang D, Henao R, et al. Convolutional neural network to identify symptomatic Alzheimer’s disease using multimodal retinal imaging. British Journal of Ophthalmology 2022;106:3:388-395.
24. Grewal DS, Fine HF, Fekrat S. Is OCT angiography useful in neurodegenerative diseases? Ophthalmic Surgery, Lasers and Imaging Retina 2019;50:5:269-273.
25. Casaletto KB, Ward ME, Baker NS, et al. Retinal thinning is uniquely associated with medial temporal lobe atrophy in neurologically normal older adults. Neurobiology of Aging 2017;51:141-147.
26. Zhang JF, Wiseman S, Valdés-Hernández MC, et al. The application of optical coherence tomography angiography in cerebral small vessel disease, ischemic stroke, and dementia: A systematic review. Frontiers in Neurology 2020;11:1009.
27. Ma JP, Robbins CB, Stinnett SS. Repeatability of peripapillary OCT angiography in neurodegenerative disease. Ophthalmology Science 2021;1:4:100075.
28. Lee T, Rivera A, Brune M, et al. Convolutional neural network–based automated quality assessment of OCT and OCT angiography image maps in individuals with neurodegenerative disease. Translational Vision Science & Technology 2023;12:6:30.
29. Li XX, Wu W, Zhou H, et al. A quantitative comparison of five optical coherence tomography angiography systems in clinical performance. International Journal of Ophthalmology 2018;11:11:1784-1795.
30. Rifai OM, McGrory S, Robbins CB, et al. The application of optical coherence tomography angiography in Alzheimer’s disease: A systematic review. Alzheimer’s & Dementia 2021;13:1:e12149.