Telemedicine is a rapidly expanding, multifaceted field that’s seeing a marked uptick in use.1 From large-scale screening programs at research institutions to the virtual visits implemented by practices across the country in response to the pandemic, telemedicine has aided triage, reduced COVID-19 transmission,2 saved some practices from closing and expanded care to underserved populations.3,4 However, its usefulness in clinical practice now is limited by the technology available to clinicians and patients, and in some cases by the clinicians and patients themselves.
In this article, telemedicine experts discuss some of the ongoing work in large-scale screening programs, and clinicians share their own telemedicine experiences during and after the pandemic.
 |
| Figure 1. The Rutgers’ mobile clinic has been screening community members for vision-threatening diseases for more than 15 years. |
Screening Programs
“Telemedicine for diabetic retinopathy is well established, much more so than for glaucoma,” says Albert S. Khouri, MD, a professor of ophthalmology and a glaucoma specialist at Rutgers New Jersey Medical School. “The form of telemedicine we’ve used for community screening dates back more than 15 years. The program has evolved along with the technology, and the protocols and algorithms we use have evolved as well.”
Dr. Khouri says the Rutgers mobile clinic recently resumed community outreach programs, after a short pause during the pandemic (Figure 1). The mobile unit, which contains a portable OCT, is driven around the state to schools, soup kitchens, community centers, churches, temples and mosques. “The program is typically advertised within the local community, and then we screen subjects for vision-threatening diseases—the big four being glaucoma, macular degeneration, DR and cataracts. We identify pathology and either refer patients to a local ophthalmologist or to the university for continuity of care.”
Screening patients with the mobile clinic is very efficient, says Dr. Khouri, who presented a study on the protocol’s time efficiency at ARVO several years ago. “We use motorized tables that elevate the equipment and screen patients standing up,” he explains. “Sitting, standing and then sitting and standing again is the most time-consuming aspect of screening, especially when dealing with elderly patients. Measuring visual acuity, IOP and obtaining anterior and posterior segment images takes about two to three minutes per subject. All the data is then transferred to a computer, and whoever is directing the operation at the time goes over the findings with the subject, or communicates with an off-site ophthalmologist, to give the patient a recommendation in real-time.”
At Stanford Medicine’s Byers Eye Institute, David Myung, MD, PhD, an assistant professor of ophthalmology, leads the Stanford Teleophthalmology Autonomous Testing and Universal Screening (STATUS) program. The Bay-Area-wide program uses artificial intelligence and telemedicine to detect referral-warranted diabetic eye disease at Stanford Medicine-affiliated primary care clinics.
Dr. Myung says that when they began the program using teleophthalmology alone, they saw a consistent improvement in the ability of the primary care clinics to increase patients’ adherence with annual retinal exams, an important quality-of-care measure in the management of patients with diabetes. “The program enabled the clinics to exceed their goal of hitting the 90th percentile for this measure, which is part of the Healthcare Effectiveness Data and Information Set (HEDIS), even in the midst of the pandemic. It was a great demonstration of how a telemedicine program can tangibly affect patient health on a larger scale.”
Dr. Myung is also a member of the executive committee of the Collaborative Community on Ophthalmic Imaging (CCOI), a group of stakeholders—including members of the U.S. Food and Drug Administration, the National Eye Institute, leading professional societies and patient organizations—that seeks to clarify the challenges, best practices, strategies and standards for ophthalmic imaging.5 “During our conference last September, we had a vibrant discourse on diseases that lend themselves well to the use of AI for image interpretation, such as ROP, macular degeneration, ocular oncology and glaucoma,” he says. “As a community, we’re considering the steps needed to bring autonomous testing and AI algorithms to critical use for these diseases. We’ll be discussing these and other topics germane to ocular imaging further at our upcoming conference in January.”
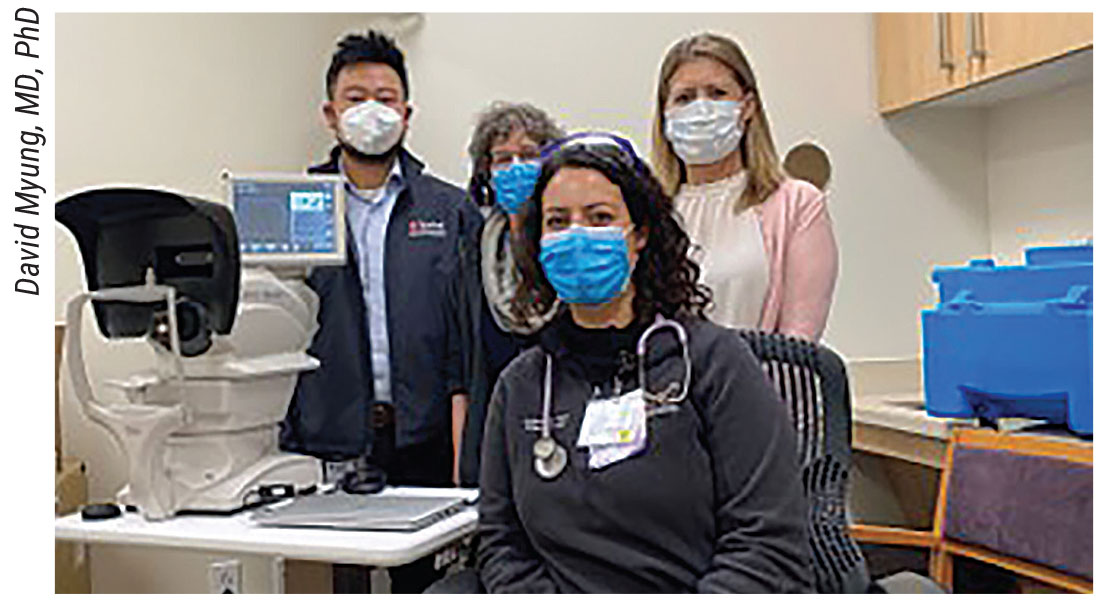 |
| Figure 2. The IDx-DR launch at Santa Clara. Stanford Medicine incorporates artificial intelligence in its Bay-Area-wide program for diabetic eye disease testing and screening. (From left to right, back: David Myung, MD, PhD, Marcie Levine, MD, Jill Terrill from Digital Diagnostics; front: Elizabeth Greksouk, NP.) |
The autonomous AI-based testing component of the STATUS program launched in December 2020. “We’ve now seen seven primary sites that use IDx-DR, an FDA-cleared AI algorithm for detecting referral-warranted diabetic retinopathy,” says Dr. Myung (Figure 2). He describes the program as an “AI-human hybrid” model where the image interpretation load is shared between the AI software and human providers at the Stanford Reading Center (STARC), which is led by his colleague, Theodore Leng, MD, FACS, an associate professor of ophthalmology. They say they’ve found that this model triages the more challenging images to retina specialists, helps more patients get screened, and ultimately enables patients to be referred appropriately for in-person care.
“Implementing AI into the system involved a highly collaborative effort between ophthalmologists, primary care providers, ambulatory care leadership, IT and cybersecurity, among many others,” Dr. Myung says. “Now, with this AI-human hybrid workflow model in place, we’re excited about what our program can do to further facilitate efficient and timely care for our patients.”
Uncovering Other Pathology
Dr. Khouri and his colleagues published a clinical study in 2020 through the New Jersey Health Foundation comparing tele-glaucoma and clinical evaluation before the pandemic, that demonstrated its potential to mitigate some clinician bias when making diagnoses.6
“We ran patients through a tele-glaucoma virtual model and collected data, including visual acuity, IOP and anterior and posterior segment imaging, which were evaluated remotely by a reader for diagnosis, management and follow-up,” he says. “In the clinic arm, patients went to a glaucoma clinic routinely as they would if they were examined at the university and got the routine standard-of-care examination, which included visual acuity, pressure, a slit lamp exam, HVF and OCT, as needed.
“We compared outcomes between the tele-glaucoma arm and the face-to-face arm, in terms of accuracy of diagnosis and treatment recommendation,” he continues. “The two arms were comparable, but there were advantages and disadvantages with each. The face-to-face visit is standard of care and demonstrated superior accuracy of optic nerve head assessment and functional data acquisition. The tele-glaucoma arm was completely lacking in HVF data, but it had the advantage of a being a quicker visit. It was significantly shorter than an office visit.”
Surprisingly, his team found that ancillary non-glaucoma diagnoses were more likely in the tele-glaucoma arm. “That group underwent non-mydriatic posterior pole digital imaging,” he explains. “We uncovered other pathology like hypertensive retinopathy or AMD drusen that were overlooked or not picked up as a diagnosis at the office visits, where the clinician knew the patient was coming in for glaucoma management and treated the glaucoma but didn’t comment on the ancillary findings picked up by tele-glaucoma imaging.” This corroborates the findings of a previously published literature review of real-time teleophthalmology and face-to-face consultation, which found that, in terms of diagnostic accuracy, tele-ophthalmology was superior in one study and comparable in six.7
 |
| Figure 3. In a study, physicians and medical students at Rutgers University used telepresence robots to deliver patient education and perform synchronous teleophthalmology visits with patients. However, users say the robots were a bit difficult to maneuver, especially if the WiFi signal was weak. |
Dr. Khouri says his study demonstrated that telemedicine for glaucoma is a viable option. “You can be very accurate in your diagnosis,” he says. “But the study also highlighted the shortcomings of tele-glaucoma, particularly with the functional aspect, which is very important. We’re not just treating pressure measurements in these patients. Functional testing—mainly peripheral vision testing or visual field testing—is paramount when discussing quality of life with these patients. That piece was missing from the telemedicine part. I’m hopeful that with all the innovation—there are some virtual reality or objective visual field tests in the works—we’ll be able to improve on this.”
Telepresence Robots
While it sounds a little futuristic, telepresence is just a fancy word for synchronous telemedicine, where doctors connect with patients in real time, as they would in the office, explains Dr. Khouri. He and his students began a telepresence robot project before the pandemic, but when Rutgers’ COVID restrictions prevented conventional means of research, the project really took off. “One of my students, Ashley Ooms, came up with the idea and executed it,” says Dr. Khouri. “She knew we had a robot that we’d used at community screening events in Florida; she used it remotely to deliver questionnaires and teaching modules to patients to find out how much they knew about glaucoma. We hoped to enhance their understanding about glaucoma and their use of topical medications.”8
The robot consists of an iPad-like screen and camera at the end of a pole that moves on a single wheel (Figure 3). A doctor can drive the robot from a smartphone, as well as raise and lower the camera and screen to be eye-level with the patient. “We can put a Snellen chart on the screen, but it’s tedious to move the robot to a specific distance from the patient,” notes Dr. Khouri. “We mostly used it for real-time communication with patients who continued to come in for glaucoma treatment, and for patient education, delivering questionnaires, collecting data and counseling subjects when an ophthalmologist wasn’t on site.
“We didn’t know what to expect when we ran the study, but we were pleasantly surprised,” he continues. “The majority of patients received it very well. Instead of just hearing an ophthalmologist through the speaker of a phone, they can see you, and the level of communication and connection is much better when it’s both audio and visual. The fact that you could navigate the robot around made it more realistic. The issue with it though, is cost, and the models are still a little clunky. We weren’t that great at driving the robot—we’d bump into things. Driving the robot also depends on your internet bandwidth. If the Wifi is spotty, it’s hard to drive until the signal is stronger.”
Pandemic-era Practice
“In ophthalmology we’re challenged and blessed with the necessity of specialized equipment,” says Brandon Baartman, MD, a cataract, cornea and glaucoma surgeon in practice at Vance Thompson Vision in Omaha, Nebraska. “When the coronavirus pandemic hit, we all had to adapt to continue caring for patients with chronic issues. Unfortunately, during the pandemic, many of these patients were still lost to follow-up, resulting in poor outcomes.”9
In early 2020, there were more unknowns about the virus, he notes. “The medical community wasn’t sure then how the virus spreads or whether it could exist on surfaces. Many of our early efforts were put in place to protect patients against some of those unknowns. At my practice, we used virtual visits for certain acute cases like red eye and for triage. We created a separate schedule in our practice management software with time slots for virtual visits.”
Initially, many practices relied on video conferencing programs like Zoom and FaceTime to reach patients—and the relaxed regulations enabled this emergency use—but most soon switched to dedicated telehealth solutions with HIPAA-compliant software. “We tried to be adaptive to the technology constraints of our patients,” says Dr. Baartman, who used Doxy.me to manage virtual visits.
“Virtual visits worked mediocrely for us at best,” he continues. “There were very few problems that patients came to us with that we could confidently treat and manage with a virtual visit. We’re a largely surgical referral practice, so we ended up seeing a lot of those patients in-person after a virtual triage. Our virtual visits slowed down over the course of 2020 and were almost nonexistent in our practice come early 2021, when we saw a decrease in COVID cases and an increase in the understanding of the virus and how it spreads. It also coincided with an increase in vaccination and vaccine availability.
“At our peak we probably did about three or four virtual visits in a day—it was challenging to instruct our team, when a patient called in, on who could receive a tele-visit and who needed to come in for an exam. When we reopened more routine care in mid-2020, we kept virtual slots open, but many patients began returning, just out of a need to leave the house and have some social interaction.”
Dr. Baartman says his practice billed only a small percentage of virtual visits, because most of them turned into triage, “which we ultimately felt was our responsibility as providers, since we often had the patient come in anyway.” He says that when his practice shut down, billing some virtual visits was better than no revenue, but with the PPP loans, ability to see patients on an emergent basis and his practice’s aggressive reopening strategy, “we certainly didn’t think the time it took to coordinate and deliver care virtually ended up being a financial factor for us.”
Here’s how some other subspecialties fared:
• Glaucoma. Though mobile units like Rutgers’ exist, that form of telemedicine is very different from most virtual visits conducted during the pandemic. “Virtual visits came about mostly during the pandemic for us,” says Dr. Khouri. “COVID really catapulted telemedicine from a niche process at select sites in the country, such as the VA system and research or academic centers, to being readily available to patients almost everywhere. But we really had no access to objective data during virtual visits. The most value was in maintaining connection with patients, reinforcing medical recommendations and making sure patients had access to their medications.”
When Dr. Khouri and his colleagues realized the shortcomings of virtual visits, they quickly set up a different protocol to create a hybrid virtual visit. This hybrid model, which they used during the heavy months of the pandemic, employed a drive-thru where patients could be evaluated by a small eye-care team for visual acuity with a Snellen card and IOP with both Tono-Pens (Reichert) and iCare handheld tonometers, which have disposable tips. “We were also able to perform handheld slit-lamp exams, but we couldn’t image the fundus with this care model,” he says. “We did virtual visits later that day.”
At ARVO and AAO in 2020, his team presented a study comparing the drive-thru and virtual visit model to just a virtual visit. “In ophthalmology, with virtual visits alone, we’re less likely to make any treatment-altering recommendations,” he says. “With a hybrid visit, we’re more likely to adjust medical treatment. There were limitations to the hybrid model, however. Patients had to drive to a separate location to get measurements. Once vaccinations picked up and things began to reopen, it didn’t make sense for the patient to drive all the way to the office and not come inside for a proper slit lamp exam.”
• Retina. Retina specialist Shriji Patel, MD, MBA, of the Vanderbilt Eye Institute says, “Telemedicine is far from where we want it to be; however, it was an excellent stopgap at the time when it was unsafe for patients to come into the office and be around other patients or providers.”
His multispecialty practice used Epic’s HIPAA-compliant telemedicine visit option. “We were able to keep it secure and safe, and patients would connect through their Vanderbilt electronic health portal so it was a seamless entryway,” he says. “We’d have the telehealth visit on our templates as a scheduled visit. Patients would be queued up for us in the virtual waiting room, and then we just clicked a button to start the visit. We used it a little for patients who we absolutely needed to get a read on but couldn’t come in, but I’m not convinced it was much more helpful than a phone call.”
Dr. Patel says that during virtual visits, he was able to deal with triage issues and assess to some degree how an eye was doing postoperatively. “This was mainly done by gathering information from the patient: how is the eye feeling, what’s your vision like? Is this superficial bleeding that’s typical in a postop course, or is there blood vessel dilation and tortuosity suggesting inflammation? Are there signs of infection—a hypopyon? These were about all we could assess from a virtual visit.”
Additionally, he says that getting used to the software was challenging for patients and providers. “We had to have IT support on hand to make sure everyone could sign in and get on the system at the appropriate times.”
Like Dr. Patel, Ken Lord, MD, of Retina Associates of Southern Utah, didn’t find telemedicine particularly useful for his subspecialty. “We limited a lot of our routine follow-ups during the height of the pandemic, and we kept seeing our injection patients, but that was about it,” he says. “We never did any telehealth. If we had patients who needed to see us for emergencies, there wasn’t much telehealth could do.”
• Cornea and cataract. As expected, telemedicine was slightly more applicable in anterior segment subspecialties; however, the lack of objective data still hindered its utility. Josh Frenkel, MD, MPH, of Wang Vision Institute in Nashville, an anterior segment surgeon, says his practice began using telemedicine during the pandemic for cataract and refractive surgery screenings.
“We continued virtual visits with Doxy.me for a while after we opened again, and our optometrists did some as well,” he says. “Patients seemed to enjoy virtual visits they could do from home. However, after a while it became challenging because virtual visits required extra time during the workday. Our clinic was busy enough that we didn’t have the capacity to keep them in.”
He says telemedicine has the potential to increase the number of refractive consults his practice does. “Right now, we have one of our patient coordinators, who’s working remotely, follow up on leads and do LASIK consults over the phone,” he notes. “That’s helped workflow efficiency, and it’s more convenient for patients—getting them that first touch-point for their initial evaluation. It’s helped weed out non-candidates. These refractive screenings have worked best with telemedicine because the age group is largely comfortable with using digital technology. LASIK also isn’t insurance-based, so we don’t have to worry about coding and reimbursement for screening calls. Your refractive coordinator might be a good person to take on this role. Any way you can save doctor time is beneficial to the practice.”
Dr. Myung has been implementing virtual care paradigms during the pandemic through the VA Palo Alto Health Care System. “The VA is a leader in virtual care tools,” he says. “We were able to implement secure video visits very quickly during the pandemic for patients who wanted it because the telehealth infrastructure was already in place.”
Prior to the pandemic, Dr. Myung piloted a remote surgical preoperative evaluation program for the VA, where pre-op evaluations were handled remotely prior to cataract surgery. “We found that we were able to do a lot of the preoperative management remotely so that the patients didn’t need to make multiple trips to Palo Alto from far away before their operation,” he says. “The proof-of-concept program worked well, with care starting with a technician-only visit for diagnostic testing and imaging, followed by a synchronous telehealth visit with an eye-care provider.”
He says this work is now part of a larger effort by the VA to expand access to care across its Veterans Integrated Service Networks through the new Clinical Resource Hub (CRH) program. “Through the CRH program, we’re working on ways to expand access to both medical and surgical care through the VA system to our veterans in more remote areas. For surgical specialties like ophthalmology, we’re focused on diagnosis and surgical decision-making via virtual care and providing surgical services when and where appropriate.”
• Pediatrics. Ken K. Nischal, MD, FAAP, FRCOphth, the division chief of pediatric ophthalmology at Children’s Hospital of Pittsburgh and medical director for digital health of the hospital, says the pandemic has been a crucial moment in American medical health delivery. “Until this public health emergency, patients had to be seen in a facility,” he says. “You couldn’t see them at home and be paid for it. The Medical Assistance Bulletin has said that no matter what happens after this emergency, home consultations will continue.”
Dr. Nischal’s hospital developed a pediatric telemedicine program in response to the pandemic. He and his colleagues published a paper detailing their implementation methodology in the Journal of American Association for Pediatric Ophthalmology and Strabismus.10 The study covered outpatient records from March 21 to April 10, 2020. Before March 21, scheduled patients were categorized into three groups: requiring 1) an in-person visit, 2) a face-to-face visit that could be postponed and 3) a consultation that could be virtual.
The ophthalmology service offered the option of a virtual visit to 237 patients and scheduled 212 visits, with 25 patients declining. Those who declined a virtual visit were offered a telephone visit, and if they still declined, they were then offered an in-person visit when it was deemed safe. The ophthalmology service completed 206 (97 percent) of scheduled visits during the study period with seven providers. A total of 43 visits were with new patients, and the other 163 were follow-ups. After an initial visit, 21 patients required a virtual follow-up an average of four weeks later, and 170 required an in-person visit an average of 4.6 months later. None of the patients needed to be seen within 72 hours. Within the hospital-wide urgent care virtual platform, a total of 290 patients were seen, with 25 eye-related visits (eye pain, conjunctivitis, edema of lid(s)), and none of these patients ended up being seen at the ophthalmology clinic, but may have been followed up elsewhere.
“We posted a video on YouTube explaining how to test your child’s vision before the telemedicine appointment using various apps,” he says. “Many parents found it difficult to download or use the apps, however. Our ophthalmic technicians would do an intake by phone the day before the appointment and ask about any changes since the last visit. Almost all of these patients were follow-ups.
“For new patients, we created an algorithm so they could be seen safely,” he continues. “In the paper, there’s a list of conditions that would require a primary in-person visit (e.g., amblyopia, optic neuritis, myasthenia) or a primary telemedicine visit (e.g., chalazion or mild conjunctivitis referred by a PCP/ED in new patients; a first post-op visit for lid laceration or strabismus surgery; or testing results for electrophysiology).”
During a virtual visit with video, the ophthalmologist would perform an external examination by instructing parents to position the child closer to the camera; assess extraocular movements with a fixation target; and simulate a cover test by asking parents to cover eyes alternately. They would then perform a risk/benefit analysis and triage patients into video management or an in-person visit.10
Dr. Nischal says telemedicine consults have helped his colleagues take weight off their in-person schedules. “All of the attendings in my division had two or three telemedicine slots during administrative time,” he says. “It’s easy to sit and do some administrative work and also do a few telemedicine appointments. You don’t feel stressed, you’re reducing your in-person visit load, and you can do telemedicine at home, if you want to. It gives you flexibility.”
However, deploying the program overall was challenging. The workflows and protocols were continuously refined, so standardized scripts were used to ensure communication consistency. Additionally, testing visual acuity at home with apps meant standardization was lacking, with no way to ensure proper testing distance or calibrate devices, and results were sometimes given in nonconventional notations.10 Examining patients without a video-enabled device was also challenging.
Despite these shortcomings, he says the virtual nature of telemedicine has helped with child attention. “Communication is better,” he says. “When they’re just focused on you, and you on them, in front of a screen, fifteen minutes is a long time. They get to ask you questions that they might otherwise not feel able to, thinking you’re too busy to answer them during an in-person visit where there are other distractions. In fact, some children with special needs are more willing to let you look at their eyes through a screen than they are in person. They don’t see it as a doctor’s exam because they’re sitting at home.
“We also saw a very low no-show rate due to the convenience of telemedicine,” he says. The scheduled clinic e-visits had a no-show rate of 3 percent in the study. “Parents love it. It saves them time, and they don’t have to take time off work.” He hopes a future paradigm will include remote visual assessment facilities with sophisticated testing equipment.
Improving History-taking
Without access to most objective data, the questions you ask your patients take on even more importance. “At the time, we didn’t have the setup to do testing remotely at other sites,” Dr. Nischal says. “We needed to ask very targeted and thorough questions. The art of history-taking has become defunct, for want of a better word, because of electronic medical records. We’re often so busy looking at the EMR that we aren’t concentrating on asking targeted questions. We also don’t get a chance to sit and talk to the patient as much as we used to.”
Here are some examples of targeted questions you might ask your patient and/or their caregiver:
- If a patient is blinking a lot, and it’s a recent development, you might ask about outdoor activity (did something get into the eye?), photophobia or air conditioning in the home. Do the eyes feel dry?
- If a patient finds it difficult to open his eyes in the morning, is he sleeping with his eyes open at night? Is a caregiver checking this? “You’re not able to examine a patient for lagophthalmos remotely,” Dr. Nischal notes.
- If you suspect a patient may have glaucoma, Dr. Nischal says sometimes he enlists a caregiver’s help. “I ask them to close their eyes and the patient’s,” he says. “I tell them, ‘Feel your eyes first and then feel his. What’s the difference?’ You have to use that kind of surrogate testing sometimes, though it’s not perfect.”
- If a patient has controlled glaucoma, you might ask, “Compared to three months ago (or since the last visit), have your eyes been watering more?” Dr. Nischal suggests. “When the patient goes outside, is she photophobic? Does she seem to be bumping into anything? When walking through doorways, is she bumping into the frame with a certain side of her body? If the patient is encountering any of these problems, I have them come in.”
A Work in Progress
 |
| Figure 4. This printable home visual acuity test, when used with a standardized protocol, was found to produce equivalent scores to standard technician-administered tests in a study published in Ophthalmology Science this year.11 |
Telemedicine technology is evolving at an impressive speed. Yet, experts say we’re still just on the cusp of what’s possible. “Telemedicine was a learning experience,” says Dr. Baartman. “I’m hopeful that it’ll evolve into a practice-enhancer for patient accessibility purposes, but we’re still waiting for the development of the necessary at-home technology to facilitate a better exam and experience for providers and patients needing virtual eye care. During the pandemic, I felt the virtual visits were a worthwhile exercise that we hadn’t found a long-term use for yet.”
“Right now, the main goal of telehealth and remote vision monitoring is to catch disease that needs to be treated as early as possible,” says Dr. Lord. He says remote vision screening and monitoring platforms for teleophthalmology will need both clinical validation and FDA approval.
There are a few already approved by the FDA, including two autonomous artificial-intelligence algorithms: IDx-DR (Digital Diagnostics) and EyeArt (Eyenuk) for detecting diabetic eye disease (see the November 2021 issue of Review); ForeseeHome (NotalVision), an at-home nAMD monitoring device and program; and Alleye (Oculocare Medical), a free mobile app for self-monitoring AMD progression. Needless to say, it’s important to caution patients against using apps for vision testing that haven’t been rigorously vetted and approved.
Here are just a few other issues that telemedicine and its users will need to address:
• The patient’s ability to use the technology correctly. “In the office, we control the exam, but with at-home monitoring, the patient is in control,” says Dr. Lord. “Most of our patients are over 70 and aren’t as adept at handling a mobile or digital interface as younger patients. We also need to be able to get a reliable interpretation of the results they give us.”
A new printable home visual acuity test for teleophthalmology was recently validated in a study published in Ophthalmology Science this year.11 The study included 209 eyes from 108 patients who had scheduled in-person outpatient clinic visits. Patients were sent a .pdf document with instructions and a printable ETDRS vision chart calibrated for use at five feet (Figure 4). The patients completed the test at home (with 98 percent reporting good ease of use); they were then measured by a technician using a standard ETDRS chart in the office. Mean adjusted VA letter score difference was 4.1 letters (90% CI, 3.2 to 4.9), which was well within the seven-letter equivalence margin, the researchers said. Average unadjusted VA scores in clinic were 3.9 letters more than scores at home (90% CI, 3.1 to 4.7), and absolute difference was 5.2 letters (90% CI, 4.6. to 5.9). The researchers said the standardized-protocol at-home test was equivalent to a standard technician-administered test in the study.
• The clinician’s ability to stay on top of compliance. “It’s a big responsibility to manage the health of a patient remotely,” Dr. Lord says. “There are a lot of compliance issues you need to be aware of, especially when it comes to managing patient data.” Be aware of licensure, state regulations, synchronous versus asynchronous visits, patient consent for telemedicine, parity laws, Medicare restrictions and HIPAA.12
• Data privacy. Any device or telehealth platform that’s gathering patient information must be HIPAA compliant, but it’s difficult to ensure this. Physicians should be careful about the service they choose. “This has to be a priority for developers,” says Dr. Lord. “Patients’ information must be private, and the servers and anything else gathering patient data must also be secure and HIPAA-compliant. The other option is to not gather data, and to have the device simply tell the patient, ‘yes, you’re fine’ or ‘no, you’re not,’ and leave it up to the patient to contact a provider if they’re not doing well.”
Before the device meets the home user, developers and regulatory bodies will also need to consider questions of data privacy for the algorithms, which need large datasets to train on. Removal of identifiable information from large datasets is difficult, and reidentification may always be a concern.13
• Establishing standards. Standards for image acquisition, image and data transfer, interpretation and encryption are all needed. “With DR, many years ago we established standards for DICOM transmission of images and how you interpret pathology for DR on a standard image,” notes Dr. Khouri. “Other diseases like glaucoma are really on a spectrum, so we don’t have standards for acquisition, transmission and interpretation of data.”
• Ensuring equity. Screening programs, in particular, have great potential in underserved communities, but screening is only one part of teleophthalmology. Virtual visits depend heavily on individuals’ access to digital technology, such as computers or smartphones with good-quality cameras, internet access and tech literacy. If the pandemic has showed us anything with regard to health-care access and remote schooling, it’s that not everyone has these. Black and Hispanic people are more likely to suffer from visual impairment and be less digitally literate, while being less likely own a smartphone or have access to quality internet.14
Dr. Nischal says video visits have been advantageous since they enable you to take a screenshot of the patient’s eye as you conduct the exam, but the quality of the image is heavily affected by the patient’s internet bandwidth. “The less well-off you are, the more likely it is that you’ll rely on cellular data, rather than a true Wifi router in the home,” he says. “The quality of what you see over cellular data isn’t as good as high-bandwidth Wi-Fi. We may be improving access, but the quality of what you access isn’t equal.”
• Counseling patients carefully about self-monitoring. With more at-home monitoring technology in development, it’ll be important for doctors to not only instruct patients on how to perform the tests, but also to counsel them about what they may find. For example, some patients use home tonometers to report their pressures, which has the benefit of obtaining IOP at several times a day, as opposed to during a single office visit. However, Dr. Baartman says he’s seen patients go over the top checking their pressures. “They become concerned with what could otherwise be a normal fluctuation in eye pressure,” he says. “Putting more technology in patients’ hands is definitely a double-edged sword. They may become too fixated on their disease.”
Dr. Khouri receives grant support from Allergan, Optovue and the NJ Health Foundation. Drs. Myung, Baartman, Frenkel, Patel, Lord and Nischal report no related financial disclosures to anything mentioned in their comments.
1. Saleem SM, Pasquale LR, Sidoti PA, et al. Virtual ophthalmology: Telemedicine in a COVID-19 era. Am J Ophthalmol 2020;216:237-42.
2. Zhou B, Zhang I, Patton A, et al. Comprehensive tele-ophthalmology screening in vision threatening diseases during COVID-19. ARVO Annual Meeting Abstract. Presented June 2021. Invest Ophthalmol Vis Sci 2021;62:1899.
3. Hark LA, Adeghate J, Katz LJ, et al. Philadelphia telemedicine glaucoma detection and follow-up study: Cataract classifications following eye screening. Telemedicine e-Health 2020;25:8:992-1000.
4. Ghazala FR, Hamilton R, Giardini ME, et al. Teleophthalmology techniques increase ophthalmic distance. Letter to the Editor. Eye 2021;35:1780-81.
5. Collaborative Community on Ophthalmic Imaging. http://cc-oi.org. Accessed Nov. 2, 2021.
6. Mintz J, Labiste C, DiCaro MV, et al. Teleophthalmology for age-related macular degeneration during the COVID-19 pandemic and beyond. J Telemed Telecare 2020. [Epub September 29, 2020].
7. Kapoor S, Eldib A, Hiasat J, et al. Developing a pediatric ophthalmology telemedicine program in the COVID-19 crisis. J AAPOS 2020;24:204-208.
8. Chandrasekaran S, Kass W, Thangamathesvaran L, et al. Tele-glaucoma versus clinical evaluation: The New Jersey Health Foundation prospective clinical study. J Telemed Telecare 2020;26:9:536-544. [Epub May 28, 2019].
9. Tan IJ, Dobson LP, Bartnik S, et al. Real-time teleophthalmology versus face-to-face consultation: A systematic review. J Telemed Telecare 2017;23:7:629-38.
10. Ooms A, Shaikh I, Patel N, et al. Use of telepresence robots in glaucoma patient education. J Glaucoma 2021;30:3:e40-e46.
11. Siktberg J, Hamdan S, Liu Y, et al. Validation of a standardized home visual acuity test for teleophthalmology. Ophthalmol Sci 2021;1:100007.
12. Sumner S, Schick DC. Telemedicine: Compliance issues during and after COVID-19. Sumner and Schick. https://www.sumnerschick.com/telemedicine-compliance-during-covid-19. Accessed Nov. 2, 2021.
13. Tom E, Keane PA, Blazes M, et al. Protecting data privacy in the age of AI-enabled ophthalmology. TVST 2020; Special Issue:9:2:36:1-7.
14. Scanzera AC, Kim SJ, Chan RVP. Teleophthalmology and the digital divide: Inequities highlighted by the COVID-19 pandemic. Eye 2021;35:1529-1531.