|
Mark Packer, MD, FACS |
Implanting multifocal intraocular lenses has always involved a trade-off: the hope of gaining spectacle independence in exchange for sacrificing a little image quality. Of course, most of these individuals are happy to be freed from wearing glasses. But if a patient has a disease such as glaucoma, the trade-off may involve additional considerations.
A multifocal IOL like the ReStor or Tecnis reduces image quality to some extent partly because of diffraction interference; these lenses only transmit about 80 percent of the incoming light. Also, multifocals divide the light between distance and near, with the ratio depending on lens design and pupil size. For example, the Tecnis multifocal uses about half of the transmitted light for distance and half for near, independent of pupil size; the ReStor ratio changes with pupil size; and refractive lenses like the ReZoom and Array function like monofocal distance lenses if the pupil is smaller than 2.8 mm, but split the light for multiple foci when the pupil is larger.
This slightly reduced image quality isn't a problem if you have a healthy, functioning fovea, but glaucoma can cause reduced contrast sensitivity due to optic neuropathy and losses in the nerve fiber layer. In advanced glaucoma, central threshold may be reduced in the perifoveal region from the normal 33 decibels into the 20s or even lower.
Therein lies the potential problem when implanting a multifocal IOL in a glaucomatous eye: You may be making current—or future—loss of vision worse, by allowing the multifocal lens to reduce image quality even further. Even if the effect is negligible at the time you implant the lens, a multifocal can shorten the length of time the glaucoma patient will maintain functional vision.
What's Best for the Patient?
Of course, some patients with glaucoma are highly motivated to achieve spectacle independence and want to try a multifocal lens. However, given the potential problem, we need to be able to counsel these patients as to whether that's advisable. Little has been written about this in the literature, so we have to use a common-sense approach.
First of all, the presence of glaucoma, even with visual field loss, shouldn't be a blanket contraindication to implanting a multifocal lens as part of refractive lens exchange or cataract surgery. Instead, we need to consider the patient's condition: Does she have mild, nonprogressive glaucoma, or more advanced or potentially progressive glaucoma?
If a patient has some mild, peripheral visual field loss but good central function, 20/20 vision and controlled pressure (on only a few medications) with proven stability over a year or two, implanting a multifocal lens is probably fine. You can reassure the patient that her pressure will probably remain controlled after the surgery (barring the risk of complications that any patient might encounter). In fact, her pressure might even be lower postop, since pressure generally is lower after lens extraction. In addition, you can offer the patient an adjunctive procedure in order to control IOP or reduce the number of medications.
The challenge arises, of course, when someone has poorly or intermittently controlled pressure, or visual field loss, particularly with decreased perifoveal threshold. In this situation, the possibility of losing functional vision earlier because of a multifocal implant becomes more of a concern. Here, three alternative options are worth considering.
First, an accommodative monofocal lens such as the Crystalens may prove a workable alternative because it doesn't reduce contrast sensitivity or affect luminance by splitting the light for multiple foci the way a multifocal IOL does. However, we know from U.S. Food and Drug Administration studies that while 80 to 90 percent of patients achieve spectacle independence with a multifocal, closer to 70 percent achieve this with the Crystalens. For this reason, the patient needs to be forewarned that he may still end up with a low-powered pair of reading glasses.
A second alternative is to go ahead and implant whichever multifocal is best suited to the patient's vision needs, despite the future risk. This may be a reasonable course of action if the patient is highly motivated to be free of glasses and really does understand that he may be making a trade-off—better vision now in exchange for possible earlier loss of functional vision. Obviously, the patient's understanding of the risk is crucial.
The third option is to implant a good aspheric monofocal lens like the Tecnis, SofPort or AcrySof IQ, chosen to offset the patient's specific corneal asphericity. A study by Roberto Bellucci, MD, showed that putting an aspheric lens in normal people produces a perifoveal threshold about 4 decibels higher than if you put in a standard spherical IOL.1
Furthermore, matching the choice of aspheric lens to the preexisting corneal spherical aberration as closely as possible will minimize the spherical aberration and generate the best quality retinal image. Although the patient may not be freed from the use of reading glasses, by increasing the image quality formed on the retina instead of decreasing it the way a multifocal does, functional vision may actually be maintained longer, instead of disappearing sooner.
What's Causing the Vision Loss?
Another concern is that some glaucoma patients undergoing cataract surgery may not achieve the level of visual clarity they expect following lens replacement. This can happen when some of the central vision loss they've been experiencing turns out to be the result of glaucomatous optic neuropathy and NFL damage, rather than being entirely the fault of the cataract. If the patient desires spectacle independence, knowing whether the glaucoma is contributing to the visual loss could be an important factor in the decision about whether a multifocal is the best lens to implant.
This kind of glaucomatous central vision loss may be readily detected by automated perimetry in a patient with a clear lens, but can be difficult to detect when a cataract impedes the view. A potential acuity meter can be used to get an approximation of visual acuity that bypasses the cataract to some extent, but this may not provide enough information to answer the question. Optic disc imaging can provide some helpful information, but out of necessity some clinical judgment must be involved as well.
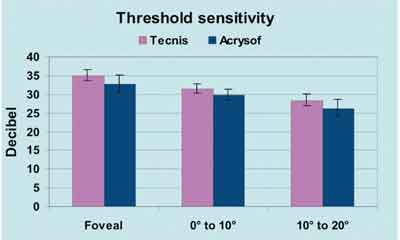 |
| In a study of luminance threshold involving 30 eyes, presented by Roberto Belluci, MD, at the 2002 ASCRS meeting, mean sensitivity was significantly higher with an aspheric modified prolate IOL than with a control spherical IOL (p=0.001). This difference could be significant for eyes damaged by glaucoma. |
For example, when one glaucoma patient with well-controlled IOP came in with a cataract complaining of vision loss in that eye, a Heidelberg Retina Tomograph scan showed no change in his disc topography. This provided some reassurance that there was at least no visible sign of optic nerve damage. His visual field showed the same glaucomatous scotomas as before, but also some increased diffuse loss of sensitivity. Since the complaint was only in the eye with the cataract, and other indications suggested that the glaucoma was stable, my clinical judgment was that the degree of diffuse loss of sensitivity on his visual field could be explained entirely by the cataract. However, I couldn't guarantee that his vision would be totally clear if we took the cataract out. (In this situation, it's important to inform the patient that his vision loss may be partly caused by progression of his glaucoma, so he may not get as much improvement after cataract surgery as either you or he would like.)
Luckily, the diffuse sensitivity loss cleared after cataract removal. Also, we combined his operation with endocyclophotocoagulation, so his postop pressure remained low without the four medications he'd been using. This patient wasn't concerned with spectacle independence, but if he had been, I would have considered a multifocal implant. I felt pretty confident that the diffuse loss of sensitivity was not caused by the glaucoma.
When Functional Vision Deteriorates
In upcoming years, as more patients are implanted with multifocals, some will undoubtedly develop glaucomatous damage or have similar visual deterioration as a result of other diseases like diabetic retinopathy or macular degeneration. When a patient like this begins to have trouble with night driving or recognizing faces at a distance—functional tasks that are highly dependent on contrast sensitivity—you'll have to decide whether explanting the multifocal and switching to a monofocal is worthwhile as a way to improve visual function.
Generally, if the capsule is intact and clear and a patient with progressive glaucoma is noticing decreased vision with a multifocal lens, you have a good argument for performing an IOL exchange, especially if you replace it with an aspheric lens that's matched to the cornea's spherical aberration. However, it will be important to consider the severity of the disease, how much of the problem may be attributable to the lens, and factors that might make surgery more risky.
Several studies have shed light on ways in which perimetry can help us determine the extent of glaucomatous damage that may be causing visual loss beyond that caused by the cataract. In general, while mean deviation may be affected by both cataract and glaucoma, pattern standard deviation provides a better indication of glaucomatous visual field loss.2,3 Also, when a central dense scotoma is present before cataract surgery in two or fewer meridians, the patient may well achieve substantial improvement in postoperative visual acuity.4
Testing contrast sensitivity might also be helpful. In general, glaucoma produces a depression in peak contrast sensitivity at the mid-range spatial frequencies (3 to 6 cycles per degree), while cataract causes diffuse depression of contrast sensitivity across all frequencies.
The YAG Capsulotomy Factor
One key consideration is the presence or absence of a YAG capsulotomy. Sometimes, if a patient complains of visual difficulty with a multifocal, the surgeon performs a YAG capsulotomy, even if the capsule looks clear. If there's any chance of a lens replacement becoming necessary later, this may not be a good choice.
Trying to do an IOL exchange when the patient has had a YAG capsulotomy is risky. Should vitreous prolapse occur, you might have to perform a vitrectomy, increasing the risk of cystoid macular edema, which would undermine vision even further. You're also risking damage to the capsular bag, which could make implantation in the bag or sulcus impossible. Suturing a lens in place or implanting an anterior chamber lens in a patient who has trabecular insufficiency could make the glaucoma worse.
Outlook for the Future
Although clinical experience suggests that these considerations relating to multifocal lenses in glaucoma patients are important, they have not yet been validated with clinical trials. Hopefully, this will change in the future. In the meantime, future multifocal lens designs that reduce spherical aberrations will likely improve outcomes for these patients.
Dr. Packer is in private practice in Eugene, Ore., and is clinical assistant professor of ophthalmology at Oregon Health & Science University. Dr. Smith is assistant professor of ophthalmology at Northwestern University in Chicago. Drs. Packer and Smith serve as consultants to Advanced Medical Optics and Bausch & Lomb, and have received travel support and honoraria from Alcon Surgical and Eyeonics.
1. Bellucci R. Humphrey and high-pass perimetries with Pharmacia Tecnis and Alcon AcrySof IOLs. Presented at the American Society of Cataract and Refractive Surgery Annual Symposium, 2002. Abstract available at http://www.ascrs.org/Meetings/abstract-search-results.cfm?id=3938.
2. Lam BL, Alward WL, Kolder HE. Effect of cataract on automated perimetry. Ophthalmology 1991;98:1066-70.
3. Chen PP, Budenz DL. The effects of cataract extraction on the visual field of eyes with chronic open-angle glaucoma. Am J Ophthalmol 1998;125:325-33.
4. Hayashi K, Hayashi H, Nakao F, Hayashi F. Influence of cataract surgery on automated perimetry in patients with glaucoma. Am J Ophthalmol 2001;132:41-6.