Like many technologies today, state-of-the-art phacoemulsification machines offer more ways of using them than most users think about or, in some cases, even understand. In this practical guide, experts advise you on what you might be missing. Find out how to optimize safety and efficiency with IOP, vacuum, aspiration, phaco-tip motion, power modes, continuous irrigation, chopping, quadrant removal, polishing, viscoelastic, infusion fluidics, ultrasound, phaco burst, phaco pulse and other functions. You’ll also learn strategies for avoiding and managing complications.
Beyond Routine: Uncertainty
Lisa Park, MD, an associate professor of ophthalmology at Columbia University College of Physicians and Surgeons in New York City, points out that most of her colleagues are pretty familiar with many of their phaco machines’ settings, but could always learn a new trick or two.
“Every cataract surgeon knows how to use the technology of phaco, of course,” acknowledges Dr. Park. “However, when it comes to the specifics of making adjustments to the settings on the machine? Surgeons typically set up parameters when they initially buy a new phaco machine. Then, because the machines and the surgeons are so good, many surgeons don’t necessarily feel a need to make any changes from that point on.”
To get the most out of phaco, Dr. Park recommends following the basic principle of “less is more.” She notes: “Remember that the primary goal during cataract surgery is to perform nuclear disassembly efficiently, using just the right amount of phaco energy,” she says. “Using too much ultrasound can result in endothelial cell loss, corneal edema and, in the worst cases, wound burn. Yet, setting the ultrasound settings too low may result in capsular bag movement and zonular stress, difficulty with disassembly and using too much irrigation fluid during needlessly long surgeries.”
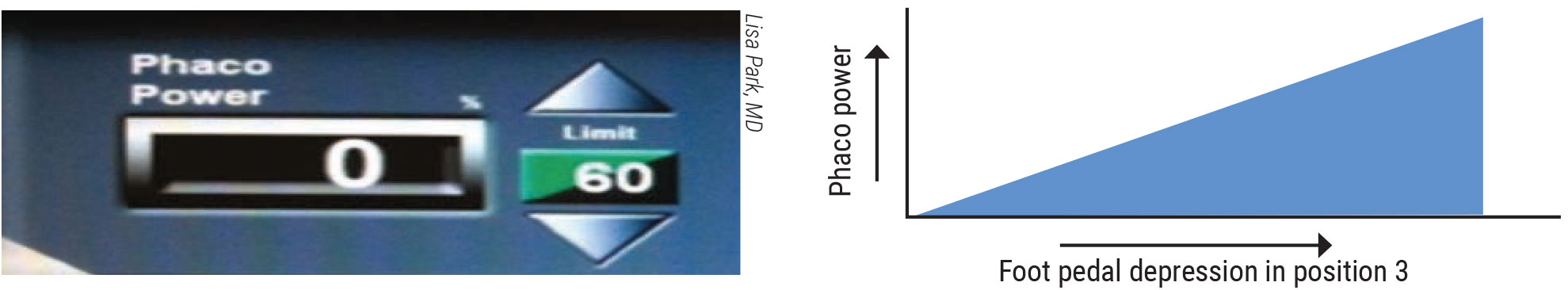 |
|
Figure 1. Continuous phaco power provides a predictable flow of energy that increases to a preset limit when you depress the foot pedal. |
She notes that longitudinal energy is the traditional phaco modality. The phaco needle moves in a forward and backward motion, creating mechanical impact in a jackhammer fashion. Cavitation bubbles appear, implode and propagate energy waves, which break up the lens material. Increasing phaco power is achieved by increasing the stroke length.
Continuous, Pulse and Burst
You can also make phaco more efficient by modifying the timing or duration of ultrasound power, according to Dr. Park. The basic power settings available include continuous, pulse and burst modes.
In continuous mode, the degree of delivered power is determined by depressing the foot pedal, which increases energy until it reaches a preset maximum, if the mode is set to “linear.” For the pulse setting, according to Dr. Park, “after each pulse of energy, no energy is delivered for a brief ‘off’ period, within a preset maximum limit. By alternating on and off periods, there’s time to cool the phaco needle and reduce heat and energy that are delivered into the eye.”
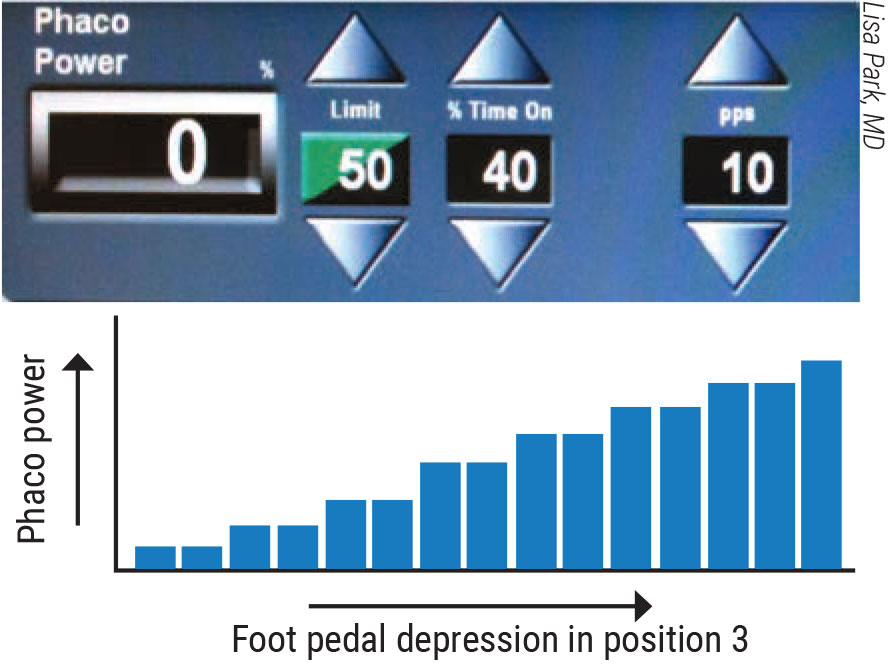 |
|
Figure 2. In phaco pulse mode, the time between pulses, known as the off time, allows the phaco needle to cool, reducing the heat and energy delivered into the eye. |
The ratio of the total phaco-on and phaco-off time is expressed as a percentage. A 50-percent duty cycle, for example, means that the power is on half the time and off the other half, according to Dr. Park. “To be clear, then, increasing or decreasing the number of pulses per second doesn’t change the total amount of energy delivered,” she continues. “If you compare a setting of 50 pulses per second to a setting of 200 pulses per second and if the duty cycle remains at 50 percent, the phaco energy will still be delivered 50 percent of the time for both settings.”
However, she adds, you can alter the duty cycle to change the on-and-off times. For example, you can program your machine to provide a 20-percent duty cycle, which results in 20 milliseconds of energy followed by 80 milliseconds of no energy in each cycle. “During the extended off time, while no energy is being delivered, the nuclear fragments can easily be aspirated,” says Dr. Park. “Therefore, you can still have 200 pulses, but the amount of energy delivered in this duty cycle would be 20 percent of continuously delivered phaco energy.”
Managing the Pulse Rate
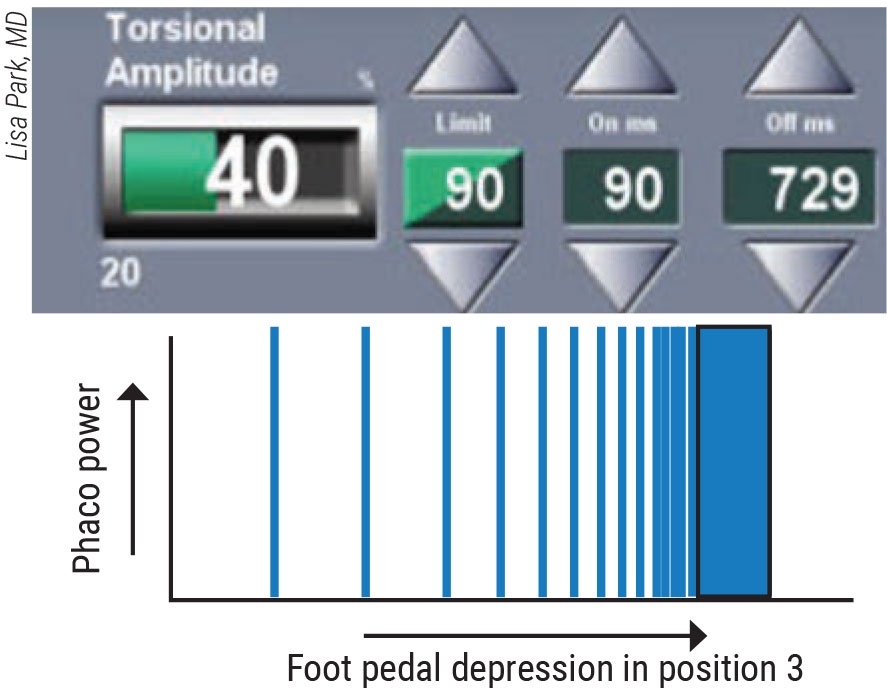 |
|
Figure 3. In the phaco burst mode, bursts of equal levels of energy are delivered more rapidly as the pedal is depressed. |
Dr. Park emphasizes selecting the right pulse rate and duty cycle for the right circumstances. During sculpting, for example, energy creates a groove and, therefore, higher pulse rates tend to work better because the narrower time intervals between pulses produce a smoother delivery of ultrasound energy. For quadrant removal, she says, a lower duty cycle tends to be a better choice most of the time because a long interval between pulses allows for the aspiration of nuclear fragments.
Burst, the third mode, also helps in special situations. “Each burst has the same power,” says Dr. Park. “The interval between each burst decreases as the pedal is depressed. The more the pedal is depressed, the shorter the off period is between each burst. In other words, the bursts of energy are delivered more rapidly as the pedal is depressed. At the maximum point of depression, the time between bursts becomes infinitely smaller and essentially constitutes a continuous delivery of energy.”
Dr. Park says the burst mode allows for “true” phaco-assisted aspiration of the lens nucleus. The vacuum and fluidics of the phaco machine are used to aspirate the cataract fragments and give small bursts of power only when necessary, she notes. “Because we can program these bursts to be as quick as a few milliseconds, we can give hundreds of tiny bursts each second,” she points out. However, in these situations, she notes that the surgeon doesn’t have linear control of the phaco power that’s delivered.
“Our ability to program timing and duration in combination with the directional modes, such as transverse and torsional phaco, can result in highly elegant control and precise ultrasound power delivery,” says Dr. Park.
Besides reducing heat and energy, “pulse and burst modes can theoretically improve followability and improve efficiency,” says James D. Auran, MD, professor of ophthalmology at the Columbia University Irving Medical Center. “Micropulse mode uses a pulse of 4 to 10 milliseconds in duration. Long pulse mode is a 30-to-60 millisecond pulse duration.”
Remember Your Parameters
Dr. Auran, also the surgical curriculum director for the Columbia University ophthalmology residency program, says intelligent use of phaco parameters is essential. “Intraocular pressure, for example, is a valuable tool,” he says. “Pressure pushes the capsular bag posteriorly and makes it taut, decreasing the risk of inadvertent capsular aspiration and rupture. Increased IOP also stabilizes the chamber, especially in the presence of high aspiration and vacuum or when leaking incisions are involved. We can minimize post-occlusion surge in these situations.1 However, be alert, because increased IOP can also cause endothelial damage2 and, of course, higher IOP is less comfortable, especially in high myopes.”
Dr. Auran points out that, according to in vitro data, increasing the IOP to as high as 110 mmHg in the Alcon Centurion system can make the machine more efficient.3 Another study found that, in the Whitestar Signature Pro system (Johnson & Johnson Vision), increasing bottle height to 110 cm increases efficiency in the peristaltic continuous mode.4 However, another study involving the Catahrex 3 phacoemulsification system (Oertli) found that bottle height had little influence on efficiency or chatter.5
Dr. Auran notes that vacuum varies when the phacoemulsifier is turned on, requiring you to use vacuum for aspiration only. “Control of the nuclear fragments with the vacuum is best achieved with the tip embedded in the nucleus,” he adds. “If your hold on a nuclear fragment is tenuous, wait until you can drag the fragment centrally, away from the capsule, to visualize an adequate amount of nucleus in front of the phaco tip. Quick taps of the tip will advance the tip deeper into the nuclear fragment, enabling a firmer hold.”
Keep in mind that tip occlusion is required to reach a preset maximum vacuum power (for example, 400 to 700+ mmHg) when using a peristaltic pump⎯but not a venturi. Dr. Auran says you can ensure the best use of vacuum to hold a lens, capsule or iris against the instrument tip. Increasing vacuum can also increase phaco efficiency in some machines, to some extent. Meanwhile, a very high vacuum setting decreases efficiency,6 but does allow for the aspiration of soft and medium pieces of the nucleus, which can be aspirated with minimal or no phaco power, notes Dr. Auran. “Harder pieces can be mashed against the tip of a second instrument,” he says. “Our choices depend on the texture of the individual fragment we’re handling.” He lists, for example:
- hold in place (lower vacuum);
- drag (slightly higher vacuum);
- hard nucleus phaco, (higher vacuum);
- soft and medium nucleus aspiration (higher vacuum).
Meanwhile, when you increase aspiration you increase phaco efficiency, Dr. Auran points out, noting that high aspiration can help with sticky material, such as the epinucleus and cortex, and for viscoelastic removal. However, high aspiration can also be risky. For example: A column of cohesive nucleus, epinucleus and/or cortex can draw the posterior capsule into the tip from 1 to 2 mm away in 300 microseconds.7 “So, avoid excessive aspiration,” he says. “Lower aspiration (20 to 24 ml/min) can help if events occur too quickly, such as when you’re removing a soft cataract, and you don’t want to pull materials toward the tip while grooving or during vitrectomy capsule polishing.7 Low aspiration can also help ensure protection of the endothelium, such as in cases of Fuchs’ dystrophy.”
Appreciating Variable Power
Dr. Auran says increasing phaco power generally increases efficiency. “But you’ll see less of an efficiency gain from incremental increases at higher powers, as well as decreased efficiency (in part due to chatter) at the highest powers in some situations,” he says. “Also, keep in mind that low power (for example, 20 percent) may be best for some situations, such as soft cataracts. Higher power can increase the risk of corneal injury, wound burn, iris damage and capsular rupture.”
Are there any other approaches to increase efficiency? One example, Dr. Park points out, is limiting power to prevent excessive heat build-up. She also urges you to use the innovations in phaco technology that augment longitudinal phaco, when indicated. These innovations “enable us to deliver power through lateral and rotational motions in two modes known as transversal and torsional ultrasound,” she says. Remember that transversal ultrasound helps emulsify the nucleus in more than one direction, increasing cutting efficiency. The main advantage of torsional phaco is increased energy efficiency, but the main disadvantage is significant tip movement. (Note that transverse and torsional phaco are proprietary. Transversal ultrasound [Ellips] is employed by Johnson & Johnson Vision in the Whitestar Signature Pro and torsional ultrasound [Ozil] is found in Alcon machines, such as the Infiniti and the Centurion vision systems.)
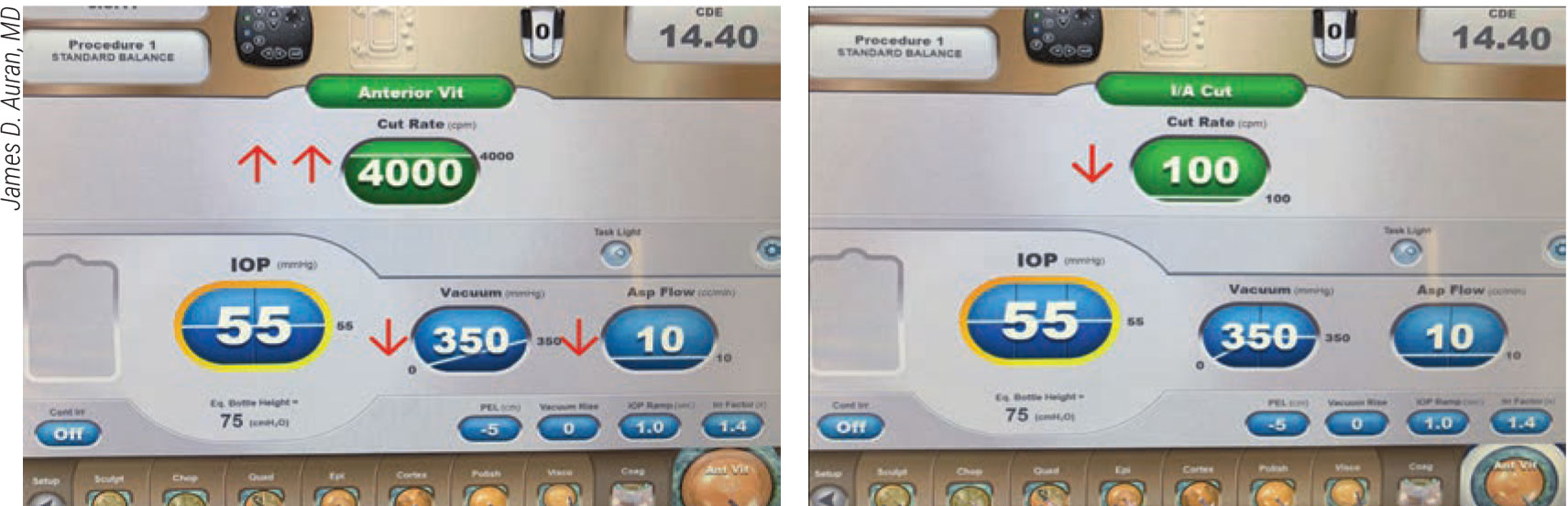 |
|
Figures 4 and 5. When performing an anterior vitrectomy during cataract surgery, James D. Auran, MD, recommends removing the vitreous at 4,000 cuts per minute and aspirating fluid in the third pedal position. For lens removal with the vitrector, make careful adjustments to draw the lens pieces away from vitreous (without aspirating the vitreous) while slowing the cut rate to 100 to 200 cuts per minute, he says. This opens the cutter port enough to produce sufficient aspiration and vacuum to engage the lens pieces. |
Meanwhile, remember that specific phaco units perform differently. For example, efficiency in the Centurion increases, using up to 100 percent of continuous power with a longitudinal-torsional balanced tip, set at IOP, 50 mmHg; aspiration, 50 mL/min; and vacuum, 500 mmHg.8 When using a torsional-Intrepid balance tip and 550 mmHg of vacuum, efficiency improves up to 60 percent.9
“When you alternate longitudinal with torsional modes, efficiency rises linearly, with an increase of longitudinal power up to 100 percent,” Dr. Auran adds. “But 60-percent torsional optimizes efficiency and minimizes chatter.10 Alternative approaches, such as manual mashing and chopping mechanisms to minimize the risk of damage, can sometimes be safer.”
The Whitestar Signature Pro, when used with longitudinal power and a 30-degree bevel straight tip, increases efficiency up to 100 percent. Efficiency is optimal at 90 percent power in the transverse mode with a 100 cm bottle height and vacuum at 600 mmHg.4 Efficiency is also optimal in the Whitestar at 90-percent power with transverse elliptical tip movement, aspiration at 50 mL/min, bottle height at 100 cm and vacuum set at 600 mm Hg.4
Avoiding Trouble
Dr. Auran explains how phaco modes can help keep your surgeries safe, efficient and responsive to the needs of varied patients.
“The sculpt mode is really for scraping off nucleus,” says Dr. Auran. “You don’t want nucleus drawn to the tip, so you should use low vacuum and low aspiration, but as much power as you need longitudinally. Unlike torsional or transverse power, longitudinal power will likely draw the nucleus toward the tip.”
The chop mode is for holding the lens, he continues. “This requires more vacuum and more aspiration, depending on the nuclear consistency,” says Dr. Auran. “The power is only employed to drill the hole to begin chopping. Some surgeons go right to quad, drawing pieces of the lens toward the phaco tip, relying on increased aspiration and vacuum and as much phaco power as needed. The torsional or transverse modes, less likely to repel nuclear particles, might be best.”
Epinuclear settings are for handling the rubbery outer layer of the nucleus, again with moderate to high vacuum and aspiration, as needed, with minimal or no phaco power and high IOP to push the capsule posteriorly, as appropriate, he notes.
“Cortex can be very adherent to the capsule, which may tend to prolapse forward into the aspiration port during cortex removal,” he says. To prevent this, elevate the IOP, pushing the capsule bag back and making it taut. “When it’s like a drumskin, it’s much less likely to be drawn into the port. When engaging and stripping cortex in the periphery, use moderate vacuum and high aspiration. Once cortex has been drawn into a safe area in the center at the iris level, increase aspiration and vacuum to aspirate cortex.”
Polish is best used with a silicone tip, according to Dr. Auran. It involves very low settings while you keep the pressure high and capsule taut. Very low vacuum and aspiration are indicated to gently remove cells while minimizing the risk of aspirating capsule material. Viscoelastic can be used to viscopolish the lens capsule. Removing viscoelastic requires the same settings as for the epinucleus, including high IOP (to keep the capsule away from your tip) and increased vacuum and aspiration to remove the viscoelastic.
When Things Go Wrong
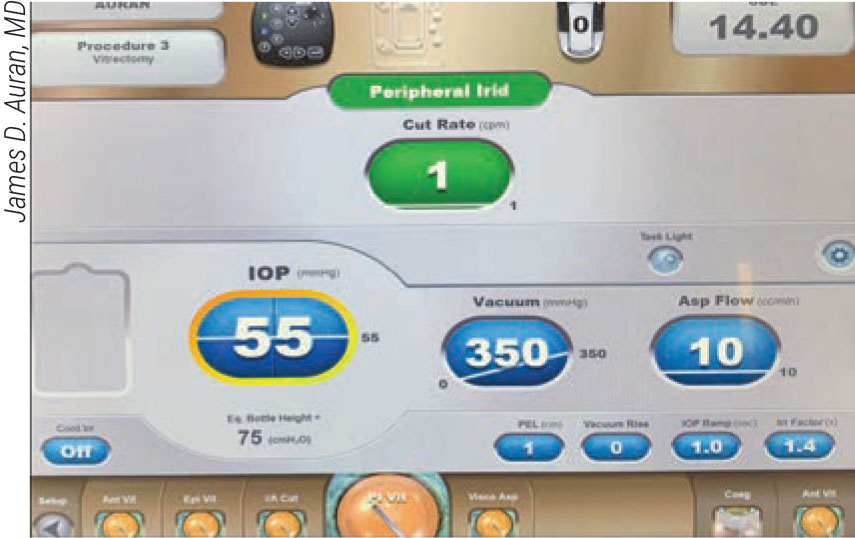 |
|
Figure 6. When performing peripheral iridotomy during cataract surgery, you’ll need to choose from a range of one cut per minute to one cut every one to two seconds. |
Dr. Auran emphasizes the need to prepare for an unexpected complication. “If vitreous gets pulled into the instrument tip, whether it’s because of a broken bag, zonular dehiscence or some other factor, if vitreous prolapses forward, your first response should always be to freeze your instrument, stop phacoemulsification and kick your pedal in the appropriate direction to reflux fluid and clear the tip,” says Dr. Auran. “If that doesn’t work, inject viscoelastic directly into the barrel of the probe or amputate the material with Chang scissors. Lower the IOP to nearly zero before withdrawing the tip. Halt all irrigation. Wiggle your sideport instrument sideways within the incision to potentiate controlled fluid egress from the sideport. Then withdraw the phaco tip quickly through the main incision, taking care not to depress the base or lift the roof of the incision while withdrawing the instrument.”
For an anterior vitrectomy, Dr. Auran repeats common words of caution: Never pull on the vitreous. “A very rapid cut rate is required, along with very low vacuum and aspiration settings,” he says. “Using 4,000 cuts per minute, the cutter is mostly closed, and nothing happens until you’re in the third pedal position and aspirating fluid. These settings allow you to apply minimal traction to the vitreous.”
For lens removal with the vitrector, Dr. Auran recommends drawing the lens pieces away from vitreous. You may need to apply the vitrector port directly against the lens piece (using irrigation only), then apply vacuum and aspiration to engage the piece, carefully pulling it into a safe zone, he says. Lens removal requires a much slower cut rate (100 to 200 per minute), allowing the cutter port to open wide enough to produce sufficient aspiration and vacuum to engage the lens pieces. “Sometimes a higher cut rate is necessary to be efficient for removal of the lens material,” he acknowledges. “But efficiency with the vitrector is extremely low for removing the material. If possible, it’s best to use viscolastic to trap the lens pieces and perform phacoemulsification in the quad setting with a very low flow.”
For a peripheral iridotomy, Dr. Auran says you’ll need to choose a range of one cut per minute to one cut every one to two seconds. “To control the cut you make, use fairly high vacuum and low aspiration,” he advises. “Make sure the port is pointed down over the iris location where you want to make the iridotomy, then make one cut to see what you’ve got.” Knowing which way to kick to get reflux and keep the iris from being drawn into the tip is also very important, according to Dr. Auran. “Know your machine,” he adds.
For a peripheral iridotomy, Dr. Auran says you’ll need to choose a range of one cut per minute to one cut every one to two seconds. “To control the cut you make, use fairly high vacuum and low aspiration,” he advises. “Make sure the port is pointed down over the iris location where you want to make the iridotomy. Also make one cut to see what you’ve got.” It’s also very important to know which way to kick your footpedal to activate the reflux mode, keeping the iris from being drawn into the tip. “Know which direction your machine requires you to kick in,” he says.
Other Potential Challenges
A few other potential challenges might include a chamber collapse in a normal-pressure eye, Dr. Auran says. “Check your equipment, looking for bad tips and problems with connections to the tips,” he says. “You can increase IOP, consider continuous irrigation or decrease aspiration. A lot of fluid escapes through the sideport, so removing or angling your sideport instrument may help. Matching the diameter of your sideport instrument with the sideport as well as hydrating or suturing the incisions may also help.”
Watch out for high phaco power, as well, he warns. “At high power, we need to continuously irrigate the incision,” says Dr. Auran. “Immediately stop phaoemulsification when the tip occludes, as indicated by a white cloud of debris appearing over the tip or an audible occlusion signal sounding. A wound burn can occur in a half a second. You can get into real trouble real fast.”
In the presence of a small pupil, Dr. Auran recommends raising the IOP to around 100 to 110 mmHg, pressing the lens diaphragm back and dilating the pupil. “This can be helpful, although it’s uncomfortable for the patient,” he says.
And for those cases that start to get out of control? “Things may be happening too fast,” Dr. Auran admits. “Lens particles are flying around, the iris and the lens capsule are snapping at you.” He recommends decreasing aspiration (flow) and power but increasing IOP. “Also consider using viscoelastic to manipulate the nucleus and cortex,” he says. “Viscoelastic is an excellent tool to use instead of your phaco tip.”
Iris prolapse is always possible, of course, but recognizing its onset can help you keep it from getting serious, according to Dr. Auran. “Don’t withdraw your instruments until you’ve addressed IOP and flow,” he says. “Like vitreous, the iris will follow the pressure gradient.
Again, halt all irrigation and wiggle your sideport instrument within the sideport to gently allow fluid egress. Once IOP drops close to zero, withdraw the phaco tip quickly (without pressing on or lifting the incison) to minimize fluid/iris egress. Then, hydraulically reposit or visco-reposit the iris, if possible. You may need iris retractors (posterior to or bracketing the main incision) to reposition the iris and hold it in the eye. Whatever you do, avoid manipulating the iris with metal, since it frays easily.”
Infusion Fluidics
One aspect of phacoemulsification that doesn’t get a lot of attention is the infusion side of the machine, says Kevin M. Miller, MD, chief of cataract and refractive surgery at the David Geffen School of Medicine at the University of California, Los Angeles.
“When the aspiration pump of a phaco machine is turned on,” he continues, “fluid is pulled from the eye. To compensate, irrigation fluid must flow passively from the BSS reservoir into the eye. Under this greater-than-zero-inflow condition, IOP no longer equals bottle height. Because there’s flow through the resistive infusion tubing, IOP drops in proportion to the amount of flow.”
It’s important to keep in mind that the infusion flow rate equals the aspiration flow rate as long as the incisions don’t leak, he continues. With aspiration rates varying throughout a procedure, IOP also fluctuates significantly, he says. While trying to operate at a nominal IOP of 55 mmHg with a variable aspiration flow rate, actual IOPs might range from a high of 80 mmHg to a low of 30 mmHg, according to Dr. Miller.
“If you started surgery with an IOP of 50 mmHg at zero inflow and ran the aspiration flow up to 60 cc/min, the IOP would drop to zero and the anterior chamber would collapse,” he says.
Options for compensating for lost pressure and avoiding a chamber collapse are varied. “The surgeon can raise the bottle height during the procedure to compensate,” he observes. “However, you might find yourself needing to raise it by several feet, which can be challenging. A negative consequence of this approach, of course, is reduced patient comfort.”
An alternative approach is injecting air into the infusion bottle. “Essentially, pressurized air injection does the same thing as raising the bottle height,” says Dr. Miller.
Manufacturers of phaco machines employ a variety of features to address fluidics issues. One solution, available through the Alcon Centurion Vision System, is the use of active infusion fluidics. A feedback system continuously measures pressure in the irrigation and aspiration lines, as well as flow rate, and commands a motor-controlled plate to respond to changes. The plate applies pressure to a bag of BBS to increase flow and releases pressure on the bag to decrease flow, enabling IOP to remain constant while irrigation and aspiration flow rates vary, Dr. Miller says.13,14
Other phaco machines do some things on the aspiration side of the system that are noteworthy. Johnson & Johnson Vision’s Whitestar Signature Pro features what it calls “on-demand fluidics,” offering a peristaltic pump for “holdability” and intraoperative control, and a venturi pump for followability and improved efficiency.15 The company says the ability to switch between pumps is made possible with the press of the footpedal.
In its Stellaris Elite phaco system, Bausch + Lomb offers what it calls adaptive fluidics, which integrate automated aspiration control with dynamic infusion compensation. This helps stabilize IOP, creating a responsive and controlled surgical environment, according to the company.
Oertli says its CatarRhex 3 relies on a system that controls flow in 0.1-ml steps. A vacuum sensor integrated into the tubing system “monitors everything without delay,” according to a company brochure. DORC says its EVA Phaco-Vitrectomy System provides enhanced fluidics. A new system called VacuFlow VTi offers, among other features, “automatic infusion compensation” for IOL stabilization and a precise flow that the company says eliminates pulsation.
Keeping It Together
Staying on top of infusion fluidics, avoiding complications, managing complications when they develop, respecting aspiration, understanding phaco parameters and mastering varied power settings are just some of the ways you can safely and efficiently optimize phacodynamics for your patients’ benefit. Keep these insights handy, and surgeons say you’ll find yourself operating more effectively and confidently.
Dr. Miller is a consultant to Alcon and LensAr and an investigator for Johnson & Johnson Vision. Drs. Auran and Park report no financial interest in any product mentioned in this article.
1. Thorne A, Dyk DW, Douglas Fanney D, et al. Phacoemulsifier occlusion break surge volume reduction. J Cataract Refract Surg 2018;44:12:1491-1496.
2. Suzuki H, Kotaro Oki K, Toshihiko Shiwa T, et al. Effect of bottle height on the corneal endothelium during phacoemulsification. J Cataract Refract Surg 2009;35:11:2014-7.
3. Jensen JD, Boulter T, Lambert NG, et al. Intraocular pressure study using monitored forced-infusion system phacodmulsification technology. J Cataract Refract Surg 2016;42:5:768-771.
4. Wright DD, Wright AJ, et al. Optimization of transversal phacoemulsification settings in peristaltic mode using a new transversal ultrasound machine. J Cataract Refract Surg 2017;43:9:1202-1206.
5. Stutz LA, Heczko JB, Bird BA, et al. Optimization of the Oertli CataraRhex 3 phacoemulsification machine. Clin Ophthalm 2019;13:633-9.
6. Ha L, Wright A, Wright DD, et al. High vacuum and aspiration on phacoemulsification efficiency and chatter for Centurion. Can J Ophthalmol 2019;54:1:136-138.
7. Davison JA. Two-speed phacoemulsification for soft cataracts using optimized parameters and procedure step toolbar with the CENTURION Vision System and Balanced Tip. Clin Ophthalmol 2015;9:1563-72.
8. Gardiner GL, Garff K, Isha Gupta I, et al. Effect of pulsing ultrasound on phacoemulsification efficiency. J Cataract Refract Surg 2015;41:11:2560-2564.
9. Boulter T, Jensen JD, Christensen MD, et al. Comparison of a torsional and a standard tip with a monitored forced infusion phacoemulsification system. J Cataract Refract Surg 2016;42:4:613-7.
10. Jason D Jensen JD, Shi DS, Robinson MS, et al. Torsional power study using CENTURION phacoemulsification technology. Clin Exp Ophthalmol 2016;44:8:710-713.
11. Bohner A, John S Peterson JS, Alex J Wright AJ, et al. Effects on phacoemulsification efficiency and chatter at variable longitudinal ultrasound settings when combined with constant torsional energy. J Cataract Refract Surg 2020;46:5:774-777.
12. Garff K, Jensen JD, Calhoon J, et al. Impact of micropulsed ultrasound power settings on the efficiency and chatter associated with lens-fragment removal. J Cataract Refract Surg 2015;41:6:1264-7.
13. Nicoli CM, Dimalanta R, Miller KM. Experimental anterior chamber maintenance in active versus passive phacoemulsification fluidics systems. J Cataract Refract Surg 2016;42:1:157-62.
14. Ting DSJ, Rees J, Ng JY, Allen D, et al. Effect of high-vacuum setting on phacoemulsification efficiency. J Cataract Refract Surg 2017;43:9:1135-1139.
15. Cahoon JM, et al. Comparison of venturi and peristaltic vacuum in phacoemulsification. JCRS 2015;41:428-432.



