
|
Untreated symptomatic VMA/VMT can lead to debilitating visual symptoms and is a risk factor for the development of full-thickness macular holes (FTMHs).6-11 Ocriplasmin is the only approved pharmacological treatment option for patients with symptomatic VMA/VMT. It is a novel truncated form of plasmin that enzymatically cleaves structural proteins at the vitreoretinal interface, leading to liquefaction and vitreous detachment.12,13 The efficacy and safety of ocriplasmin was demonstrated by the Phase III MIVI-TRUST Study program that showed that overall, a single intravitreal injection of ocriplasmin (125 µg) resulted in a higher proportion of patients achieving VMA resolution (26.5 percent) at day 28 versus placebo (10.1 percent).
A separate analysis of the Phase III trial data identified positive predictors of VMT resolution at day 28 including absence of an epiretinal membrane, VMA diameter ≤1,500 µm (focal adhesion), presence of FTMH ≤400 µm in width, phakic lens status and age <65 years (See Figure 1).14 (Ray S. Independent baseline features predictive of pharmacologic VMA resolution in the Phase III ocriplasmin clinical program. Presented at AAO Retina Subspecialty Day, Chicago, 2012.)
Real-world Experience
Recent reports have confirmed the improved rates of VMT resolution in the real world when following these positive predictors of response (See Table 1). At the Cole Clinic, 47 percent (eight of 17) of our first patients to receive ocriplasmin demonstrated VMA resolution, and 80 percent (4/5) had closure of their FTMH.15 Patients with absence of ERM and VMA ≤1,500 µm had resolution rates of 50 percent and 62 percent, respectively.15 At the Retina Institute in St. Louis, 64 percent (14/22) of patients achieved VMA resolution, and 33 percent (one of three) of patients achieved macular hole closure. Experience from the Bascom Palmer Eye Institute in Miami showed a total of 33 percent (11/33) of patients achieving VMA resolution, with 36 percent (four of 11) achieving FTMH closure post injection with ocriplasmin. (Fortun, J. Paper presented at: 17th Annual Club Vit Meeting, June 28 to July 2, 2014. Beaver Creek, Colo.)
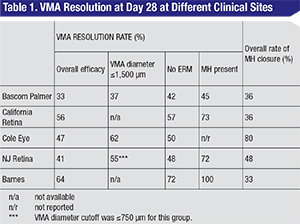 |
In summary, real world clinical results from these large practices show improved efficacy over the clinical trial results can be achieved with optimal patient selection.
Ocriplasmin Safety
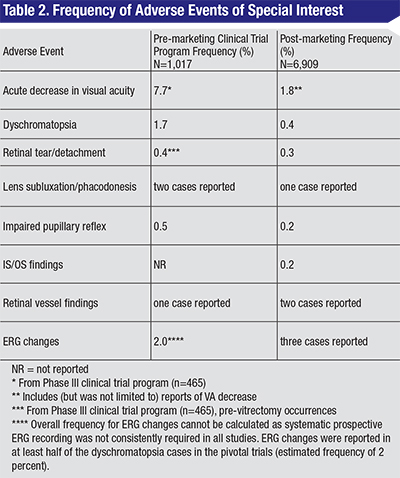
Safety data from the Phase III clinical trials demonstrated that ocriplasmin was generally well tolerated when administered as an intravitreal injection, and most adverse events were ocular and mild or moderate in severity.14 A collection of AEs were identified as adverse events of special interest by the American Society of Retina Specialists Therapeutic Safety Committee based on their clinical relevance. (Hahn P, et al. IOVS 2014;55: ARVO E-Abstract 2209.) The ASRS TSC was commissioned to monitor post-marketing drug- and device-related adverse events, utilizing periodic aggregate safety reports consisting of post-marketing reports and clinical trial data. The TSC identified different categories of AEs of interest, which included vision function changes (visual acuity alterations, dyschromatopsia and electroretinogram changes); anatomic retinal findings (retinal/macular edema [preferred term for subretinal fluid] and ellipsoid zone changes); and other safety findings including retinal tears/detachments, lens subluxation/phacodonesis, impaired pupillary reflex and retinal vessel findings (See Table 2). (Hahn P, et al. IOVS 2014;55: ARVO E-Abstract 2209.)
Visual Function Findings
The most common visual adverse events from the Phase III clinical trial program were vitreous floaters (16.8 percent) and photopsia (11.8 percent), which are known to be associated with PVD.14 Blurred vision and visual impairment were also reported in 8.6 percent and 5.4 percent of patients.14 Acute decreases in visual acuity (defined as >two line decrease) were observed in 7.7 percent of patients during the clinical trial program by day seven. The time to onset was one to two days after injection and had a median time to resolution of 14 days. A total of nine patients from the clinical trial program still had decreased visual acuity at month six, which in most cases was due to VMT or macular hole progression, or stemmed from vitrectomy complications. The reported frequency of visual impairment, according to the second periodic benefit-risk evaluation report for ocriplasmin (PBRER 2) was 1.8 percent, which included the following MedDRA terms: visual impairment; metamorphopsia; scotoma; vision blurred; visual acuity reduced; visual acuity reduced transiently; visual acuity tests abnormal; visual field defect; blindness (transient); visual brightness; halo vision; and loss of visual contrast sensitivity. Dyschromatopsia was noted in 1.7 percent of patients in the clinical trial program and in 0.4 percent from post-marketing experience.14 Of the seven events with follow-up outcomes in PBRER2, time to onset was rapid at ≤one day with the majority (57 percent) of the cases achieving resolution or resolving. ERG changes occurred frequently with dyschromatopsia. ERG changes were reported in at least half of the dyschromatopsia cases in the pivotal trials (estimated frequency of 2 percent) but systematic prospective ERG recording was not consistently done in all studies. ERG changes included decreased a and b wave amplitudes with time to onset of <one week. Only one of the Phase II trials, the MIVI-008 trial, had baseline and post-injection ERGs done in a subset of patients who received ocriplasmin. In this subset of patients who had baseline and post-injection ERGs, five of 13 (38.5 percent) showed abnormal changes from baseline, and all showed resolution by last follow-up. There were three cases of ERG changes reported from the post-marketing experience, with two exhibiting isoelectric responses. (Ocriplasmin 2nd Periodic Benefit-Risk Evaluation Report. ThromboGenics NV. December 19, 2013.) Overall, ERG changes were generally transient (median time to resolution of six months) and correlated with dyschromatopsia. (See endnotes for additional citation information.)
 |
Anatomic Findings
The visual function changes were also associated with anatomic changes. Outer segment ellipsoid zone (IS/OS junction) changes were not identified during the clinical trial program because time-domain OCT was used, while during the postmarketing period, the reported frequency was 0.2 percent (15 cases of photoreceptor alterations). In our patients, the frequency of this outer retinal change was considerably higher. We saw it in 41 percent of patients. The loss of the OS ellipsoid zone occurred by day five on average with a median time of resolution of 29 days.15 Subretinal fluid was temporally associated with ellipsoid zone layer disruption. From the Phase III clinical trial program, 5.4 percent ocriplasmin-treated patients had SRF with a median time to onset of eight days. (See endnotes.) From a post-marketing study, the SRF average time to onset was 4.5 days and average time to resolution was 30 days.15 Overall, the ellipsoid zone and SRF changes were transient.
Other Safety Findings
Lens subluxation/phacodonesis occurred in two cases in the clinical trial program and one case was reported during the post-marketing period. Retinal tears/detachments occurred in 1.9 percent of patients in the clinical trial program. Pre-vitrectomy, retinal tears/detachment occurred in two patients (0.4 percent) in the ocriplasmin group and one patient (0.5 patient) in the placebo group. The incidence was 0.3 percent during the post-marketing period. Impaired pupillary reflex occurred in 0.5 percent of patients in the clinical trials and 0.2 percent during the postmarketing period. For the postmarketing cases, the time to onset was 0 to five days with resolution in three cases, two reported to be resolving, three cases reported as unresolved, and resolution status unknown in five cases. The frequency of retinal vessel alterations was low, with only one case reported in the clinical trial program and two post-marketing cases. These findings included retinal vessel attenuation or vasoconstriction. The one clinical program case resolved but the resolution status of the cases from the postmarketing period is unknown and was ongoing at the time of the report. This collection of adverse events was low in frequency in both the clinical trial program and the post-marketing experience. (The data presented in this section is from the Ocriplasmin 2nd Periodic Benefit-Risk Evaluation Report. ThromboGenics NV. December 19, 2013.)
Ocriplasmin is the only approved pharmacological treatment for patients with symptomatic VMA including FTMH. Increased overall VMA resolution rates have been reported from postmarketing experiences at multiple centers. Positive predictors of VMA resolution include focal VMA, presence of FTMH and absence of epiretinal membrane. These baseline characteristics should aid physicians in selecting patients who are likely to gain the most benefit from ocriplasmin treatment. Safety findings were generally consistent between the clinical trial program and the postmarketing experience. While numerous hypotheses have attempted to explain the observed anatomic and functional changes, more investigations are warranted to fully understand the phenomenon. Real-world experience shows the correlation between the anatomic and functional changes and the patient symptomatology. It is therefore important to set appropriate patient expectations about the clinical course post ocriplasmin injection. REVIEW
Dr. Kaiser practices at the Cole Eye Institute. He is a consultant to Alcon, Novartis, Thrombogenics and Allegro.
Some data cited from ophthalmologymanagement.com/thrombogenics.aspx. Additional data from: Meyer J, Shah G, Blinder K, et al. Early evolution of the vitreomacular interface and clinical efficacy after ocriplasmin injection for symptomatic vitreomacular adhesion. Submitted for publication.
1. Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology 2013;120:2611-2619.
2. Sebag J. Anomalous posterior vitreous detachment: A unifying concept in vitreo-retinal disease. Graefe’s Arch Clin Exp Ophthalmol 2004;242:690-698.
3. Hikichi T, et al. Course of Vitreomacular Traction Syndrome. Am J Ophthalmol 1995;119:55-61.
4. Odrobina D, et al. Long-term evaluation of vitreomacular traction disorder in spectral-domain optical coherence tomography. Retina 2011;31(2):324-31.
5. John V, et al. Clinical course of vitreomacular adhesion managed by initial observation. Retina 2013;0:1-5
6. Steel DH, Lotery AJ. Idiopathic vitreomacular traction and macular hole: A comprehensive review of pathophysiology, diagnosis, and treatment. Eye (Lond) 2013;27 Suppl 1:S1-21.
7. Stalmans P, Duker JS, Kaiser PK, et al. OCT-based interpretation of the vitreomacular interface and indications for pharmacologic vitreolysis. Retina 2013;33(10):2003-2011.
8. Spaide RF, Wong D, Fisher Y, Goldbaum M. Correlation of vitreous attachment and foveal deformation in early macular hole states. Am J Ophthalmol 2002;133:226-229.
9. Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol. 1988;106(5):629-639.
10. Saito Y, Hirata Y, Hayashi A, Fujikado T, Ohji M, Tano Y. The visual performance and metamorphopsia of patients with macular holes. Arch Ophthalmol 2000;118:41-46.
11. McDonnell PJ, Fine SL, Hillis AI. Clinical features of idiopathic macular cysts and holes. Am J Ophthalmol 1982;93:777-786.
12. Gandorfer A, Rohleder M, Sethi C, et al. Posterior vitreous detachment induced by microplasmin. Invest Ophthalmol Vis Sci 2004;45(2):641-647.
13. de Smet MD, Valmaggia C, Zarranz-Ventura J, Willekens B. Microplasmin: Ex vivo characterization of its activity in porcine vitreous. Invest Ophthalmol Vis Sci 2009;50:814-819.
14. Stalmans P, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular hole. N Engl J Med 2012;367:606-615.
15. Singh RP, Li A, Bedi R, et al. Anatomical and visual outcomes following ocriplasmin treatment for symptomatic vitreomacular traction syndrome. Br J Ophthalmol 2014;98(3):356-360.



