Intraocular Lenses
Nighttime is the Right Time for a New IOL
Johnson & Johnson Vision recently announced availability of the presbyopia-correcting intraocular lens, the Tecnis Symfony OptiBlue IOL powered by InteliLight technology. The company says that the extended depth-of-focus lens expands presbyopia correction to more patients and joins the Tecnis Synergy IOL, a hybrid lens designed for spectacle independence, in the company’s InteliLight portfolio.
The company explains that InteliLight is a combination of three Johnson & Johnson Vision proprietary technologies: a violet-light filter; echelette design; and achromatic technology. The technology was first introduced in the Tecnis Synergy IOL.
The company says the violet-light filter blocks the shortest wavelengths of light that produce the most light scatter, which it says helps mitigate halo, glare and starbursts, and minimizes visual disturbances when driving at night. The echelette design helps reduce light scattering and halo intensity, making it easier to see digital devices, according to J&J. And the lens’s maker says the achromatic technology corrects chromatic aberration for better contrast day and night and helps give good vision at various distances. The company says the new lenses are aimed to be especially useful in terms of low-light performance and providing contrast.
The Symfony mitigates the effects of presbyopia by providing an extended depth of focus, J&J explains. Compared with an aspheric monofocal IOL, the lens provides improved intermediate and near visual acuity, while maintaining comparable distance visual acuity.
The lenses in the portfolio, Tecnis Synergy IOL and Tecnis Symfony OptiBlue IOL, are also available in Toric II versions. To learn more about the InteliLight portfolio, you can visit www.jnjvisionpro.com/intelilight.
Dry Eye
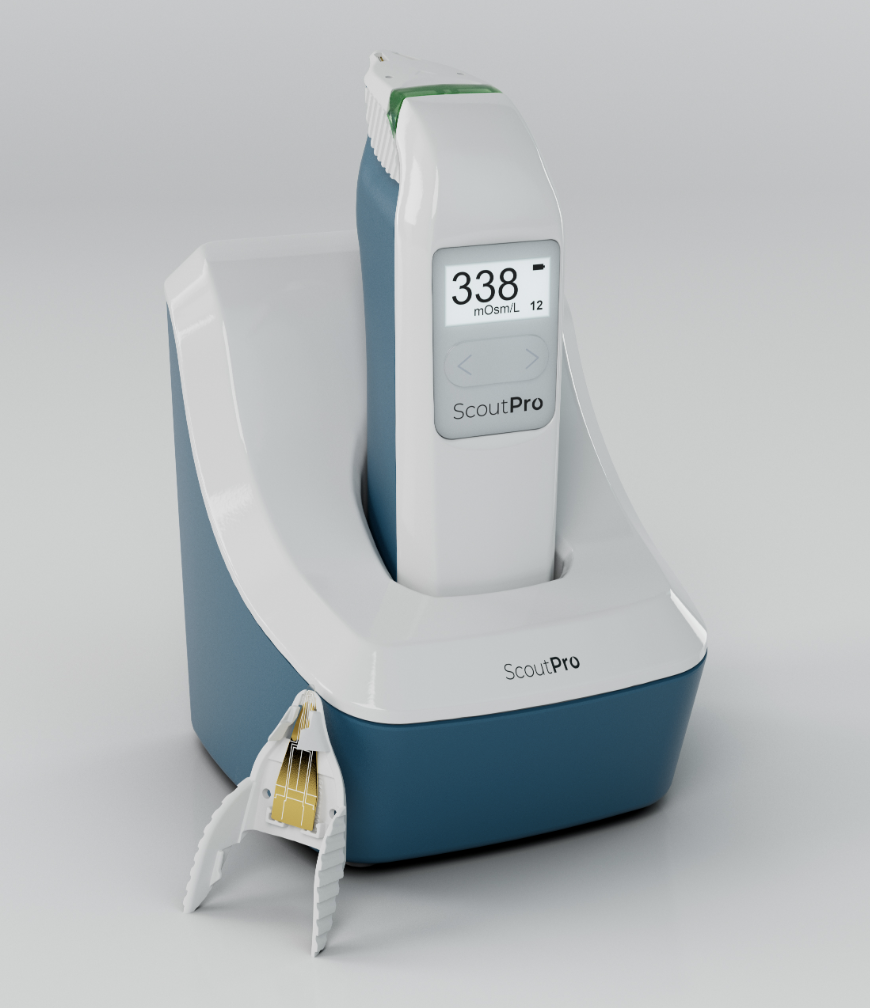 |
Trukera Medical, formerly known as TearLab, says its ScoutPro osmolarity system helps increase the convenience and efficiency of dry-eye screening.
Peforming objective tests for patients experiencing possible signs or symptoms of dry eye can help them receive timely and proper treatment, the company says. One metric to help diagnose and determine the severity of the condition is tear osmolarity. Several osmometers exist in the United States, but the ScoutPro deviates from its competitors as the first handheld version, according to the company. The device’s maker says that the ScoutPro enables both nanoliter volume sample collection and analysis to be performed from anywhere in the practice and offers quick test results “in the palm of your hand.”
The device is rechargeable with a battery life of eight hours. The charging base takes up less shelf space than some others and comes with an optional wall mount, Trukera says. The top of the ScoutPro uses what the company calls “VeriLyte technology” for specimen collection and analysis. The small screen on the device displays results shortly after each test and can store the recent scores. Trukera’s website also notes that the test cards are interchangeable with those in the first-generation TearLab osmolarity system.
For more information, visit trukera.com.
Amblyopia
Treating Amblyopia at Home
Eye patching for amblyopia is associated with a handful of adverse effects in children, including skin irritation, low self-esteem and noncompliance. In response to the desire for alternative treatments, NovaSight recently announced the FDA clearance of its new eye-tracking-based amblyopia treatment device, called CureSight. Developers say the device, designed for at-home use, helps amblyopic eyes learn to work simultaneously while a video of the child’s choice is streamed through the red-blue treatment glasses.
The treatment works by blurring the center of vision of the image shown to the strong eye, encouraging the brain to complete the image’s fine details and consequently training both eyes to work as a team, according to the company. Children are required to complete four months of treatment, with a minimum of 18 hours per month. The device’s cloud-server connection allows for remote monitoring of treatment reports by the patient’s eye-care provider via a web portal.
The company also says that the treatment can be billed through three CPT codes, which will perhaps make it accessible to a broader range of patients.
For more information, visit nova-sight.com.
Retinal Surgery
Cryosurgery in the Palm of Your Hand
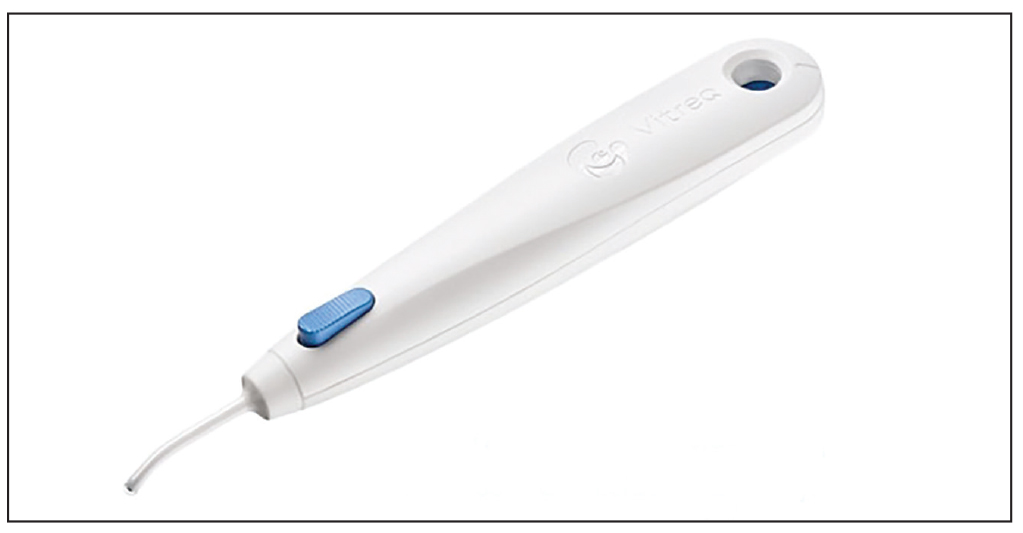 |
| BVI says its new cryo-treatment device, CryoTreq, is easy to use since it requires no service or maintenance. |
CryoTreq, a new product for treating retinal tears and detachments from BVI, debuted this year at the American Academy of Ophthalmology Meeting in Chicago for U.S. surgeons.
The cryo-based product is a handheld, stand-alone, single-use device for minimally invasive ab externo cryosurgery. BVI says it requires no external connections to equipment, gas tanks or power and doesn’t require any service or maintenance. The device is hand-controlled with a single button that activates it. Its probe reaches temperatures as low as -88 degrees Celsius and cryogenic temperatures within four to six seconds.
To learn more, visit cryotreq.bvimedical.com.
Vision Testing
Heru Adds Dark Adaptation
Heru’s wearable AMD vision testing platform has a new add-on modality to help clinicians catch early signs of age-related macular degeneration in their patients—a dark adaptation test.
The non-invasive test takes about four and a half minutes for the rapid exam and about 20 minutes for the extended exam, the company says. The test is billable to insurance with a national reimbursement average of $58.83, according to Heru. The company also notes that it’s co-billable with visual fields, optical coherence tomography, fundus imaging and/or office visits, and has multiple supported ICD-10 codes. The wearable device also includes contrast sensitivity, visual field and color vision tests.
For information, visit seeheru.com/technology.
Drugs
Anesthetic Gel Approved
Iheezo (chloroprocaine hydrochloride ophthalmic gel) 3% was recently approved by the FDA for ocular surface anesthesia. Harrow and Sintetica say the sterile, single-patient-use, physician-administered, preservative-free gel was shown to be safe and effective in three human clinical trials. In one study, effect was achieved in about one to one and a half minutes and provided sufficient anesthesia to perform a surgical procedure lasting 22 minutes, on average. The company points out that none of the patients in this study required supplemental anesthesia to complete the procedure. They also note that the single-use packaging may decrease risk of infection and medication errors associated with communal eye drops. The commercial launch is expected ahead of the 2023 ASCRS Meeting in San Diego.
For information, visit harrowinc.com.
Compounded Antibiotic to Debut
ImprimisRx is launching a new compounded antibiotic called Fortisite—which combines tobramycin 1.5% and vancomycin 5%. As part of the company’s Patient Access Program, ImprimisRx says that it will offer a 100-replacement guarantee for any expired 503B Fortisite product. The formulation can last for up to 180 days when kept refrigerated at a temperature of 5 degrees Celsius, according to a company press release.
The antibiotic is now available for order by patients through the ImprimisRx 503A pharmacy, the company says. Physicians will be able to stock Fortisite in their clinics once it’s available through the ImprimisRx 503B outsourcing facility, which is expected to happen in the first half of 2023.
For information, visit https://sf.imprimisrx.com/s/fortisite.com.
A Lucentis Alternative
Coherus BioSciences announced the commercial availability, beginning last month, of Cimerli (ranibizumab-eqrn), a biosimilar product “interchangeable” with Lucentis (ranibizumab injection) for all of Lucentis’ approved indications. Cimerli is contraindicated in patients with ocular or periocular infections or known hypersensitivity to ranibizumab products or any of the excipients in Lucentis and Cimerli. Hypersensitivity reactions may manifest as severe intraocular inflammation, Coherus says.
Cimerli is available through specialty distributors for $1,360 and $816 per single-dose vial for the 0.5 mg and 0.3 mg dosages, respectively.
For information, visit cimerli.com.