Glaucoma, like many other diseases, is subject to circadian rhythms. Studies that have tracked intraocular pressure curves throughout the 24-hour cycle have found that the "snapshots" of IOP we obtain in the office setting do not tell the whole story. And some of our long-held assumptions about nocturnal IOP have turned out to be inaccurate.
These findings have an effect on the drugs we choose to treat glaucoma. Clinicians must be aware that yesterday's gold standard medical treatment, beta-blocker therapy, does not control IOP at night, while some newer ocular hypotensive medications control IOP throughout the 24-hour cycle. Specifically, the prostaglandin analogues have been shown to flatten the circadian IOP curve nicely.
The recognition that IOP control throughout the 24-hour cycle is important for our glaucoma patients is a significant paradigm shift for today's ophthalmologists. A growing body of evidence supports two new concepts in glaucoma: One, that fluctuations in IOP may affect the progression of glaucomatous damage; and, two, that nocturnal IOP is not what we once thought it was. This article reviews some of the recent studies regarding these issues and examines their implications for the design of glaucoma medical regimens.
Do Fluctuations Affect Progression?
Elevated IOP is a known risk factor for glaucoma and for progression of glaucoma; the higher a patient's IOP, the greater the risk of progression. Increasingly, data suggest that fluctuation in IOP throughout the day, even in the so-called "normal" range, may also be a risk factor for progression. Several studies suggest the need to limit IOP fluctuation.
In one study, subjects used a home-tonometry device to perform IOP self-checks throughout the day.1 The study divided a group of 64 subjects in half based on the range of fluctuation in their IOP measurements. The subjects with the largest range of IOP fluctuation (5.4 mmHg) had almost six times greater risk of glaucoma progression within five years than those with the smallest range of fluctuation (3.1 mmHg).
In another study of 55 patients with pseudoexfoliation glaucoma, researchers found that those in the highest quintile of diurnal fluctuation in IOP had the highest risk for progression of visual field damage.2 In this study, IOP readings were taken only during office visits, so they are daytime IOP snapshots rather than diurnal curves. Still, the variability of IOP over multiple visits correlated with risk of disease progression.
Another group of researchers looked retrospectively at data from 401 patients in the Advanced Glaucoma Intervention Study to identify risk factors for progression of visual field loss.3 They found that greater IOP fluctuation was an independent risk factor for progression. For every 1 mmHg increase in IOP fluctuation over a follow-up period of seven years, risk of progression of visual field damage increased 30 percent. Although this prospective study was not designed to identify risk factors for glaucoma, these findings seem to support those of other studies cited here.
In a study of 24-hour IOP curves, patients who were known to have progressive glaucoma were checked into a hospital and monitored at time points throughout the day and night.4 No difference was seen between the mean 24-hour IOP and the IOP measured previously during patients' office visits. However, the peak 24-hour IOP measurements were on average 5 mmHg higher, and in some cases as much as 12 mmHg higher, than IOPs measured during office visits (See Figure 1). In addition, peak IOP measurements occurred outside of office hours more than half the time. As a result of the study findings, clinical management was changed in 80 percent of patients, according to the authors.
A more recent study involved 408 eyes of glaucoma patients with low IOPs (<18 mmHg) after phacotrabeculectomy, with follow-up of three to 13 years.5 Subjects were divided into those with open-angle glaucoma and those with angle-closure glaucoma, and according to the magnitude of standard deviation of their IOP measurements. The researchers found that, even though IOP was less than 18 mmHg at all times, patients whose standard deviation was 2 mmHg or greater had a three times greater risk of progression of damage (30- vs. 10-percent risk) than those with deviations of less than 2 mmHg.
Some studies did not find the same link between IOP fluctuation and visual field progression. One included patients with ocular hypertension and assessed in-office IOP curves taken every three months.6 IOP fluctuation was found to be linearly related to mean IOP level; that is, fluctuations were larger in subjects with higher IOP. But IOP fluctuation was not an independent risk factor for developing glaucomatous visual field loss.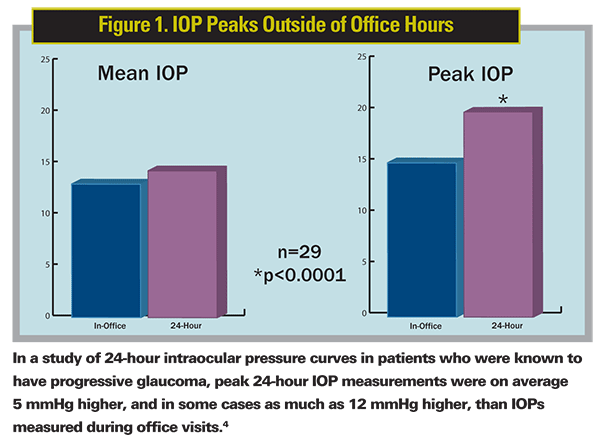
Similarly, IOP fluctuation was not identified as an independent risk factor in analysis of data from the Early Manifest Glaucoma Trial.7 The authors of that study noted that their finding "conflicts with some earlier reports." Their analyses did not include post-progression IOP values, which they noted would be biased toward fluctuation because of the initiation of more intensive treatment.
So it is not yet clear whether patients who fluctuate within the normal range have an increased risk of progression of glaucoma that is independent of the peak or mean IOP in the two groups. However, these studies remind us that IOP levels vary throughout the day, and there is more to a patient's IOP than what we can measure in the office. The highest pressures often occur outside of clinic hours, and our choices of glaucoma medications should take this into consideration.
Nighttime IOP: Flattening the Curve
Based on these studies, IOP fluctuation even during office hours may have an effect on glaucoma progression. But what about IOP at night? An increasing body of evidence shows that nocturnal IOP may be a more important factor in glaucoma than was previously recognized.
Researchers working with Robert Weinreb, MD, at the sleep lab facility at the
The accepted wisdom had been that IOP is lower at night; after all, aqueous production decreases during the nocturnal hours. But we now know that this is not the case. Given this new information, it is imperative that we rethink our approach to glaucoma therapy. Rather than being satisfied if a patient's IOP is normal during office hours, we must now be sure that we flatten the circadian curve as much as possible by lowering IOP at night.
A number of studies have now shown that certain classes of ocular hypotensive drugs control IOP at night better than others. Specifically, the prostaglandin analogues seem to be the most effective in flattening the circadian IOP curve.
Nicola Orzalesi, MD, in
The same group later used a similar study design to compare the circadian IOP patterns of the three commercially available prostaglandin analogues—latanoprost, bimatoprost and travoprost—in a crossover study involving 24 glaucoma patients and 20 with ocular hypertension.11 They found that all three were effective for controlling round-the-clock IOP.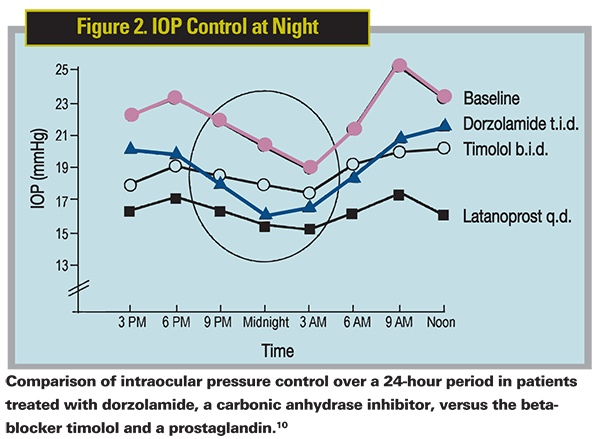
Another group compared the nocturnal IOP-lowering ability of latanoprost to that of a once-daily timolol gel.12 They found that this prostaglandin lowered IOP at all time points measured (every two hours around the clock), while the nocturnal curve of timolol closely followed the untreated baseline curve. There was essentially no therapeutic effect with the beta-blocker at night.
A similar effect was found with travoprost; in the same sleep-lab setting, researchers showed that travoprost lowered IOP both during the day and at night compared to untreated baseline.13 With IOP measured every four hours over the 24-hour period, the ocular hypotensive effect of travoprost was sustained throughout the nighttime, when untreated IOP was highest. Furthermore, IOP lowering after omission of one to two doses of the medication was sustained in the nocturnal period.
Another group further evaluated the ability of travoprost to blunt the circadian IOP curve.14 In a pilot study in 21 patients, travoprost 0.004% was administered once daily for two weeks after an initial washout period. After the final dose of travoprost at 8:00 p.m. on day 14, IOP was measured every four hours for 36 hours, and then at 60 and 84 hours after the last dose. IOP was significantly reduced from baseline at all points measured. There was minimal fluctuation in IOP (3.6 mmHg) throughout the period measured. In fact, the amplitude of fluctuation observed with travoprost in that study was as low as the amplitude of fluctuation in normal (nonglaucomatous) eyes.14
Valuable Lessons
These studies provide several valuable lessons for the management of glaucoma. They suggest that IOP fluctuations may have an important effect on progression of glaucomatous damage, although this issue is not yet settled. They show that prostaglandins provide IOP lowering efficacy with the least circadian variation in IOP. And they show that prostaglandins can lower nocturnal IOP better than the former gold standard class of hypotensive medication, beta-blockers.
Ophthalmologists must keep these lessons in mind when designing medical therapy regimens for their glaucoma patients.
Dr. Budenz is a professor of ophthalmology, epidemiology and public health at the Bascom Palmer Eye Institute, University of Miami School of Medicine.
1. Asrani S, Zeimer R, Wilensky J, Gieser D, et al. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma 2000;9:134-142.
2. Bergeå B, Bodin L, Svedbergh B. Impact of intraocular pressure regulation on visual fields in open-angle glaucoma. Ophthalmology 1999;106:997-1004.
3. Nouri-Mahdavi K, Hoffman D, Coleman AL, Liu G, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology 2004;111:1627-1635.
4. Hughes E, Spry P, Diamond J. 24-hour monitoring of intraocular pressure in glaucoma management: A retrospective review. J Glaucoma 2003; 12:232-236.
5. Hong S, Seong GJ, Hong YJ. Long-term intraocular pressure fluctuation and progressive visual field deterioration in patients with glaucoma and low intraocular pressures after a triple procedure. Arch Ophthalmol 2007;125: 1010-1013.
6. Bengtsson B, Heijl A. Diurnal IOP fluctuation: Not an independent risk factor for glaucomatous visual field loss in high-risk ocular hypertension. Graefe's Arch Clin Exp Ophthalmol 2005;243:513-518.
7. Bengtsson B, Leske MC, Hyman L, Heijl A; Early Manifest Glaucoma Trial Group. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology 2007;114:205-209.
8. Liu JHK, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci 2003;44:1586-1590.
9. Mosaed S, Liu JH, Weinreb RN. Correlation between office and peak nocturnal intraocular pressures in healthy subjects and glaucoma patients. Am J Ophthalmol 2005;139:320-324.
10. Orzalesi N, Rossetti L, Invernizzi T, Bottoli A, Autelitano A. Effect of timolol, latanoprost, and dorzolamide on circadian IOP in glaucoma or ocular hypertension. Invest Ophthalmol Vis Sci. 2000;41:2566-2573.
11. Orzalesi N, Rossetti L, Bottoli A, Fogagnolo P. Comparison of the effects of latanoprost, travoprost and bimatoprost on circadian intraocular pressure in patients with glaucoma or ocular hypertension. Ophthalmology 2006;113:239-246.
12. Liu JH, Kripke DF, Weinreb RN. Comparison of the nocturnal effects of once-daily timolol and latanoprost on intraocular pressure. Am J Ophthalmol 2004;138: 389-395.
13. Sit AJ, Weinreb RN, Crowston JG, Kripke DF, Liu JH. Sustained effect of travoprost on diurnal and nocturnal intraocular pressure. Am J Ophthalmol 2006;141:1131-1133.
14. Dubiner HB, Sircy MD, Landry T, Bergamini MV, et al. Comparison of the diurnal ocular hypotensive efficacy of travoprost and latanoprost over a 44-hour period in patients with elevated intraocular pressure. Clin Ther. 2004;26:84-91.