When it comes to treating narrow angles, the recommendations have changed over the last few years. Today, observation is a reasonable option to consider for primary angle-closure suspects (PAC-S) in addition to prophylactic laser peripheral iridotomies, and clear lens extraction is more widely accepted for treating primary angle-closure (PAC) and primary angle-closure glaucoma (PACG).
Timely diagnosis of narrow angles is crucial. Common risk factors for primary angle closure, according to the Academy’s Preferred Practice Pattern,1 include Asian descent, hyperopia, older age, female sex, short axial length, thick and anteriorly positioned crystalline lens. Dark-room gonioscopy should always be performed to verify the diagnosis of PAC and monitor response to treatment. Ultrasound biomicroscopy and anterior segment optical coherence tomography are useful for diagnosing angle closure.
Here, I’ll review two trials that have shaped our approach to primary angle closure treatment and discuss key anterior segment optical coherence tomography parameters and landmarks to aid diagnosis.
 |
Shaping Practice Patterns
Here’s an overview of the studies behind these changes:
The Zhongshan Angle-Closure Prevention (ZAP) Trial2 was conducted in 889 Chinese primary angle-closure suspect patients in 2019. In each patient, one eye received a peripheral iridotomy under the superior lid and the other eye was observed. Because of concerns for safety, these patients were tightly monitored and treated if any evidence of peripheral anterior synechiae, elevation of intraocular pressure, or other symptoms of angle closure were observed. In clinical practice, one might be a little more permissive than ZAP’s primary endpoints, which included IOP >24 mmHg at two visits, one clock hour of PAS in any quadrant, or an episode of acute angle closure. Secondary endpoints included visual acuity, IOP, total angle width on gonioscopy, limbal anterior chamber depth and adverse events with LPI or on follow-up.
This study’s population had several interesting, but not unexpected, baseline characteristics. Eighty-three percent of patients started with four quadrants of closure. The average axial length was 22.5 mm. Most patients had mild hyperopia.
Interestingly, no benefit of either LPI or observation was seen at three years. Because ZAP subjects didn’t reach primary endpoints in the initial 36 month timeframe, the trial was extended out to 72 months to determine a difference between LPI and observation. This tells us that it’s safe to watch primary angle-closure suspects, as long as they have access to care and are able to report problems.
At six years, iridotomy resulted in a 50-percent reduction in relative risk for conversion to primary angle closure with 2 percent (19/889 eyes) of the LPI group and 4 percent (36/889 eyes) of the control group reaching a primary endpoint. Only four cases (0.4 percent) of acute angle closure in three patients were observed in the entire study. One case was bilateral, so that patient was likely predisposed in some way to acute angle closure as both the treated and untreated eye were affected. Six participants (1 percent) of the LPI group experienced a pressure spike of >30 mmHg after the procedure that resolved. Much more steroid was used in this trial than is typically used in U.S. clinical practice—dexamethasone 0.1% hourly x 24 hours, then q.i.d. for one week postop.
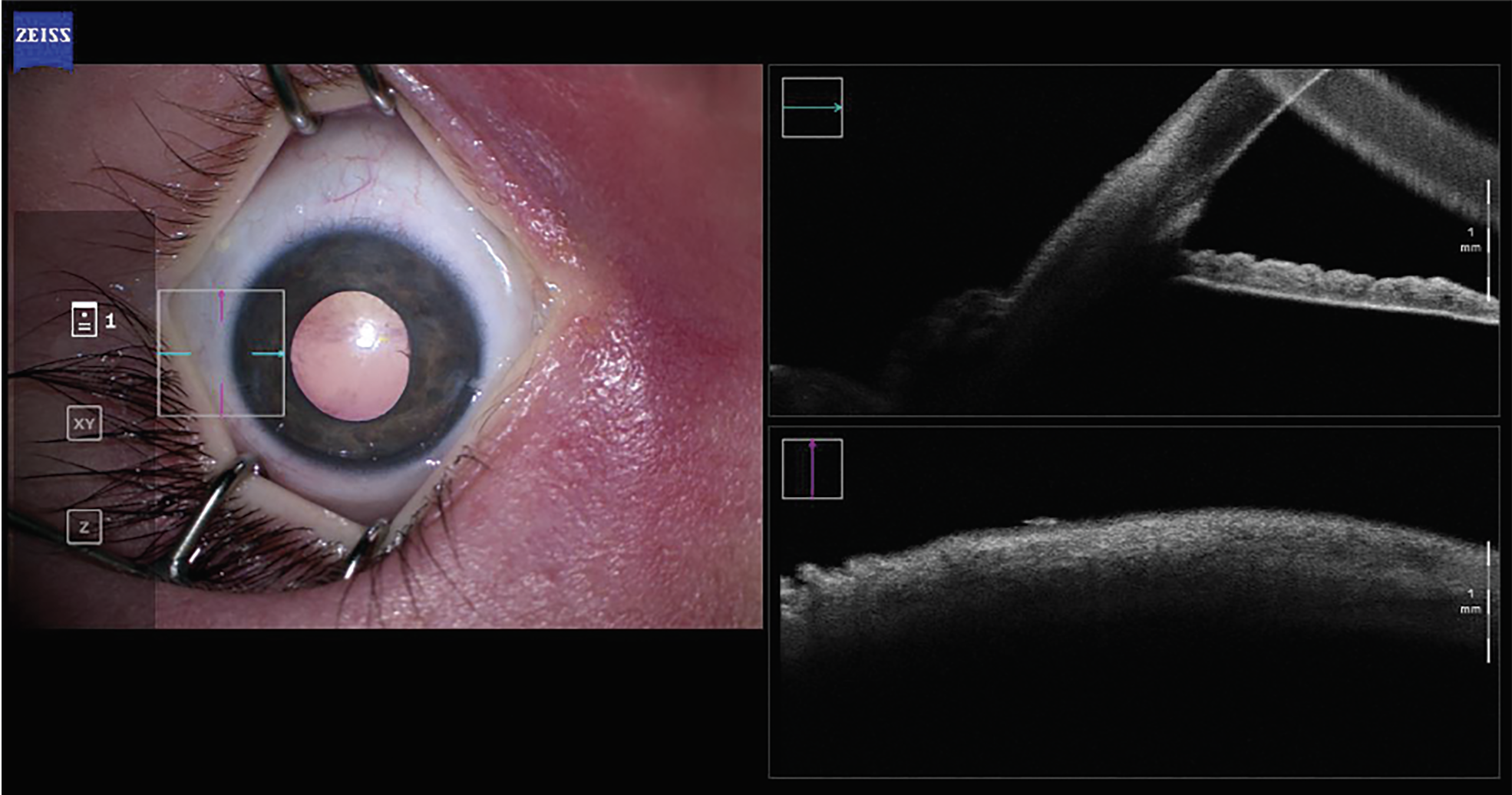 |
| Figure 1A. An intraoperative AS-OCT image of the native angle of a pediatric patient with Sturge-Weber syndrome. Pre-goniotomy, both BELL and the trabecular meshwork are visible. |
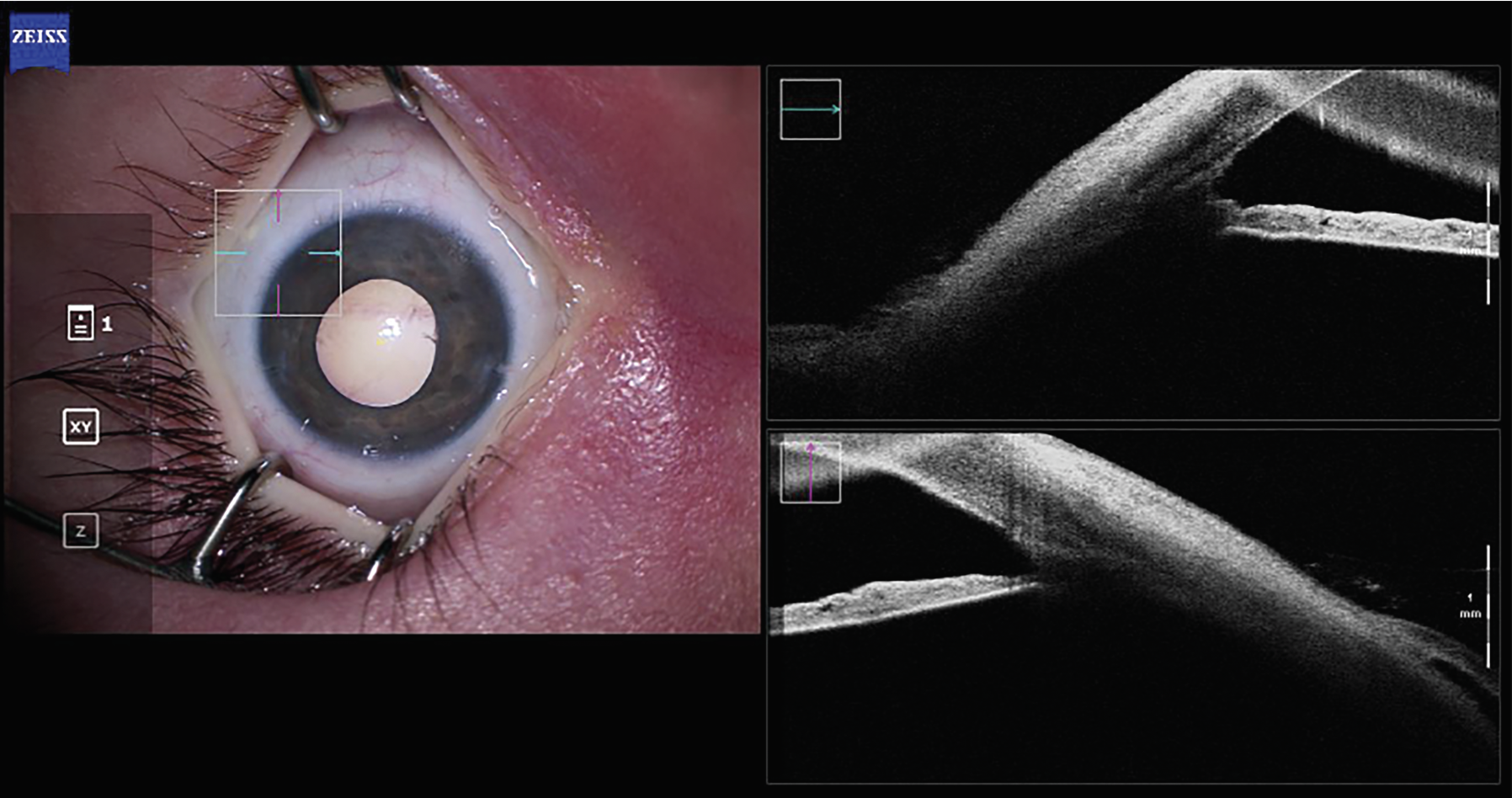 |
| Figure 1B. An intraoperative AS-OCT image of the same patient, after goniotomy. BELL is visible, while the trabecular meshwork has been excised. |
The findings from ZAP demonstrated that LPI decreased the relative risk of angle closure by half, but because the absolute risk of progression to primary angle closure was exceedingly small (4 percent), the authors concluded that, in a Chinese population, it isn’t justifiable to perform LPI in all primary angle-closure suspects. This trial demonstrated that narrow angles don’t change as quickly or as drastically as we once thought. Observation should now be considered an alternative to LPI for primary angle-closure suspects who have access to care. Nevertheless, LPI has prophylactic advantages in reducing the risk for and/or preventing acute angle closure crisis.
The EAGLE Trial3 was conducted at 30 eye hospitals in five countries. Participants were 15 years and older, didn’t have visually significant cataracts, and were newly diagnosed with primary angle closure (IOP >30 mmHg) or frank primary angle-closure glaucoma (IOP >21 mmHg and demonstrable visual field or optic nerve changes). Both clear lens extraction (n=208) and LPI (n=211) groups were similar, with 30 percent Chinese ethnicity; approximately 40 percent primary angle closure and 60 percent primary angle-closure glaucoma; a mean baseline IOP of 30 mmHg and a mean axial length of 22.5 mm.
Unlike ZAP, this trial didn’t differentiate between appositional and synechial closure, so there wasn’t a good baseline reference of who actually had PAS vs. appositional closure that could be opened up with compression or intervention. But because the EAGLE group had higher baseline pressures and the population skewed more toward primary angle-closure glaucoma, we might assume there was greater PAS burden at baseline in this population.
This study is notable for its inclusion of a quality-of-life questionnaire as a primary endpoint in a glaucoma study—the European Quality of Life 5-Dimensional Questionnaire (EQ-5D), which includes mobility, self-care, usual activity, pain or discomfort, and anxiety or depression. This study was among the first major trials in glaucoma to include this, with LiGHT being the other notable one. IOP was the other primary endpoint.
The questionnaire scores and IOP significantly favored clear lens extraction at 36 months. (Coincidentally, this is likely why ZAP chose a 36-month primary endpoint.) The EAGLE subgroups that reached statistical significance for both of these endpoints were Chinese ethnicity, primary angle-closure glaucoma, and worse baseline visual acuity. Secondary endpoints achieving statistical significance at 36 months were the NEI VFQ-25 and Glaucoma Utility Index scores, the number of medications (60 percent of the clear lens extraction group was on zero medications vs. 21 percent of the LPI group), and visual acuity.
Interestingly, the authors found that the need for additional glaucoma surgery was much lower in the clear lens extraction group than in the LPI group. Only one patient (0.4 percent) in the clear lens extraction group required trabeculectomy vs. 24 patients (11 percent) in the LPI group that required surgery to control pressures: 16 lens extractions (performed for pressure control; phaco for visually significant cataracts was excluded); six trabeculectomies; one iStent and one tube shunt.
Ultimately, the EAGLE study found that initial clear lens extraction was superior to LPI in primary angle closure or primary angle-closure glaucoma. It also identified a reduced need for further medical and surgical intervention to control IOP in the clear lens extraction group as well as improved quality-of-life measures. The only disadvantage was cost. Over the 36-month time period the group analyzed, clear lens extraction wasn’t deemed as cost-effective. However, when the authors modeled it out over a longer period of time on a population-based level, they found that clear lens extraction was more cost-effective than leaving patients with LPIs alone.
Based on these two studies, primary angle-closure suspects can be observed as an alternative to an LPI, and clear lens extraction may be considered for managing primary angle closure and primary angle-closure glaucoma.
Anterior Chamber Parameters
AS-OCT is able to detect narrow angles much earlier than we’re able to in the clinic. Though UBM and AS-OCT devices use different technology, may of the parameters used to analyze the anterior chamber are the same. There are three categories that these parameters fall into:
1. Linear parameters such as angle opening distance (AOD), irido-trabecular contact (ITC) and anterior chamber depth (ACD);
2. Two-dimensional parameters, such as trabecular iris space area (TISA); and
3. Experimental three-dimensional parameters such as trabecular iris circumference volume (TICV), which is an integrated 360-degree measurement of TISA.
The scleral spur landmark is a key reference point for defining several of these parameters. ITC, TISA and TICV are all defined at a set distance—500 µm/750 µm—from the scleral spur landmark. While the scleral spur landmark position currently needs to be manually confirmed, several companies are working toward accurate, automated identification of it.
But what are the thresholds for each of these parameters for detecting open or closed angles? To answer this question, my colleagues and I conducted a study in 2016 to validate AS-OCT parameters.4 We compiled images from four prospective studies (n=189 eyes), including gonioscopically confirmed open or narrow-angle patients and evaluated Youden optimal threshold values for each parameter using a training set with three random sets of 40 eyes each. We repeated this process 500 times per set and then applied optimal thresholds to a testing data set after a boot-strapping procedure to ensure accuracy. In total, 69 samples were tested to evaluate each parameter for sensitivity, specificity and kappa agreement between observers.
We evaluated AOD (mm) and TISA (mm2) at 500 µm and 750 µm from the SSL at each of the four quadrants; TICV (µL) at 500 µm and 750 µm; ITC length (mm) at each of the four quadrants; extent of ITC (degrees); and ITC area (mm2). All of the absolute values of the parameters we assessed were able to distinguish between open and narrow angles (all p<0.001). However, nine parameters had very high sensitivities with no false negatives. These included:
• AOD500 @ temporal
• AOD500 @ nasal
• AOD500 @ inferior
• AOD750 @ nasal
• AOD750 @ inferior
• TISA500 @inferior
• TISA750 @ inferior
• TICV500
• TICV750
Overall, the best agreement with the gonioscopy (highest kappa) was the linear measurement AOD750 inferiorly (0.91), followed by the three-dimensional TICV500/750 (0.86). The fewest angle misclassifications also occurred with those same two parameters: AOD750 inferiorly (3) and TICV500/750 (5, 5). All were false positives, meaning that the angles were mistakenly classified as narrow rather than open. AOD750 is a parameter available on any UBM or AS-OCT model. The least accurate parameter was ITC length.
A New Gonio Landmark
While looking through reference data images, my colleagues and I realized that many studies were lumping in a landmark on AS-OCT with the trabecular meshwork that we in our group called the trabecular meshwork shadow. Seen on AS-OCT, it appears to cup the trabecular meshwork and run into Schwalbe’s line at the insertion of the cornea. We delved deeper to find out what it was and how often it’s visible on imaging.5
We retrospectively reviewed 303 angles of 153 horizontal images from two-dimensional angle analysis scans (Cassia SS-1000). (The mean participant age was 51.5 years, and 64 percent of patients were female, and 66 percent of patients were white.) AS-OCT images were evaluated by masked readers. We used logistic regression to analyze several potential influential factors including age, sex, race, intraocular pressure, gonioscopy grade, angle location and history or presence of surgery on the visibility of the trabecular meshwork structures.
The trabecular meshwork was found in 73 percent (220) of angles and Schlemm’s canal was seen in 40 percent (120) of angles. The outer border of our mystery landmark, which we termed Band of Extracanalicular Lamina (BELL), after Nicholas P. Bell, MD who figured out the surgical correlate, was observed in 95 percent (288) of angles.
We believe that BELL represents a surgical landmark used in performing surgery for congenital glaucoma or traditional canaloplasty in an adult. In these procedures, you must cut down and dissect very deeply into the sclera. As you approach the limbus overlying Schlemm’s canal, there’s a compressed band of scleral fibers. These highly uniform, compressed fibers reflect light differently than the surrounding tissue on AS-OCT. BELL is visible on AS-OCT, gross pathology and histopathology staining.
The outer border of BELL was more visible in white patients than Asian patients (p=0.02), and in eyes with a Spaeth goniotomy grade of E vs. A (p=0.02). The trabecular meshwork and Schlemm’s canal were more visible in temporal angles (81 percent and 49 percent, respectively) than in nasal angles (64 percent and 30 percent, respectively; both p=0.001). Schlemm’s canal was more visible in open angles (43 percent) than narrow angles (27 percent; p=0.02). We verified each of these structures in a pathologic sample from enucleated eyes.
One of the challenges with UBM and AS-OCT is that the scleral spur landmark must be identified and confirmed manually each time and being a few pixels off can mean the difference between an open or a closed angle. We found that even when the scleral spur landmark or the trabecular meshwork aren’t apparent, we can almost always see the outer edge of BELL. From this landmark, we can qualitatively extrapolate where the trabecular meshwork is as well as its relationship to the iris. BELL provides a quick, qualitative sense of the angle anatomy.
In 2015, Mani Baskaran, MD, of the Singapore Eye Research Institute, and colleagues demonstrated in a prospective study that baseline AS-OCT predicts gonioscopic closure, where narrower angles on AS-OCT were associated with an increased likelihood of progressing clinically at four years.6 In the risk stratification, they found that 41 percent of patients with four quadrants of closure on AS-OCT at baseline, despite being open on gonioscopy, progressed to gonioscopic closure in four years. For patients who had only one quadrant of closure on AS-OCT, just 5 percent progressed to clinical closure on gonioscopy in the same time frame.
In the clinic, if you can’t do gonioscopy on a patient for whatever reason, BELL can be used to help qualitatively figure out whether the patient’s angle is narrow or open. This landmark could have future utility in population-based screening for narrow angles.
Dr. Blieden is an associate professor of ophthalmology, specializing in adult and pediatric glaucoma at the Cullen Eye Institute, Baylor College of Medicine and Texas Children’s Hospital in Houston. She has no related financial closures.
Dr. Singh is a professor of ophthalmology and chief of the Glaucoma Division at Stanford University School of Medicine. He is a consultant to Alcon, Allergan, Santen, Sight Sciences, Glaukos and Ivantis. Dr. Netland is Vernah Scott Moyston Professor and Chair at the University of Virginia in Charlottesville.
1. Gedde SJ, Chen PP, Muir KW, et al. The Glaucoma Preferred Practice Pattern Panel. Primary angle-closure disease preferred practice pattern, 2020.
2. He M, Jiang Y, Huang S, et al. Laser peripheral iridotomy for the prevention of angle closure: A single-centre, randomised controlled trial. Lancet 2019;393:1609-1618.
3. Azuara-Blanco A, Burr J, Ramsay C, et al. Effectivneses of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): A randomised controlled trial. Lancet 2016;388. [Epub October 1, 2016].
4. Melese EK, Chan JD, Blieden LS, et al. Determination and validation of thresholds of anterior chamber parameters by dedicated anterior segment optical coherence tomography. Am J Ophthalmol 2016;169:208-217.
5. Crowell EL, Baker L, Chuang AZ, et al. Characterizing anterior segment OCT angle landmarks of the trabecular meshwork complex. Ophthalmology 2018;125:7:994-1002.
6. Baskaran M, Iyer JV, Karayanaswamy AK, et al. Anterior segment imaging predicts incident gonioscopic angle closure. Ophthalmology 2015;122:12:2380-2384.