It’s not uncommon for glaucoma patients to experience difficulty performing everyday tasks as a result of low vision, or visual acuity less than 20/40 in the better-seeing eye. They may struggle to see steps and changes in terrain, read menus or spot cars parked on the roadside at night. They’re not alone. In fact, low vision affects approximately 7 million individuals in the United States, and the rates of low vision among the elderly are increasing. It’s been estimated that the number of new cases of low vision and blindness each year will more than double in the next 30 years.1
Though glaucoma is the culprit behind a large share of these low-vision statistics, a review of literature highlights that it’s not very common for glaucoma patients to use vision rehabilitation services. Here, I’ll discuss why we should recommend these services to our patients, why vision rehabilitation is currently underutilized, how to perform low-vision evaluation, and which interventions are currently available to patients.
Why We Should Treat
Vision impairment has significant negative effects on patients’ quality of life. Studies have reported that low vision may lead to a loss of independence, medication errors, increased risk of falling, social isolation, increased depressive and anxiety disorders, and increased mortality.2-5 Patients with low vision most often complain of difficulty with reading, driving and mobility. Regarding the last, it’s been reported that 49 percent of glaucoma patients struggle with steps, 42 percent with shopping and 36 percent with crossing roads.6
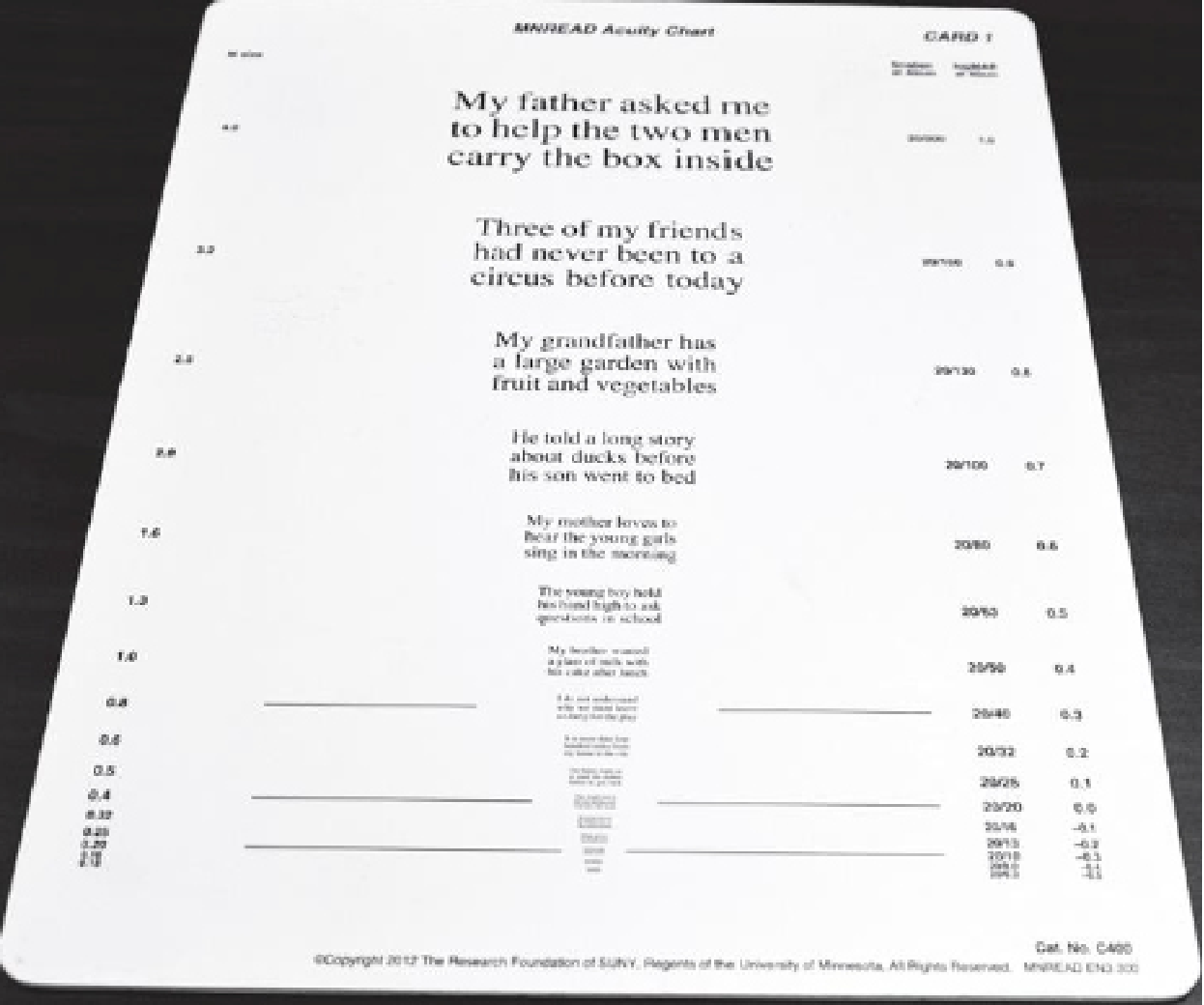 Figure 1. The Minnesota Low-Vision Reading (MNREAD) Chart can be used to determine the relationship of a scotoma to fixation in foveal-sparing scotoms. Figure 1. The Minnesota Low-Vision Reading (MNREAD) Chart can be used to determine the relationship of a scotoma to fixation in foveal-sparing scotoms. |
Even milder cases of glaucoma warrant attention for potential vision rehabilitation down the road. The Collaborative Initial Glaucoma Treatment Study reported that more than 25 percent of newly diagnosed glaucoma patients self-reported blurred vision and dark and light adaptation difficulties. Visual field testing showed only moderate correlation with these symptoms.7
Fortunately, vision rehabilitation can help improve patients’ quality of life. Patient-reported outcomes indicate improvements in daily living and emotional well-being, such as reading ability, visual motor skills, mobility, safety, independence and overall quality of life.8 Clinically meaningful improvements in reading, emotional well-being and functional independence have also been reported three to six months after initiating certain therapies.9 Clearly, it's worth referring glaucoma patients to vision rehabilitation early on to optimize their remaining vision and improve their overall quality of life.10,11 One promising approach is computer-based vision rehabilitation training for glaucoma patients. This therapy has been shown to significantly increase patients’ accuracy at detecting stimuli in high-resolution perimetry (p=0.007) and lead to faster reaction times (p=0.009) vs. visual discrimination placebo training.12 The study reported that repetitively activating areas of residual vision and areas along the visual field borders resulted in increased detection sensitivity of stimuli and visual field defect improvements. The investigators proposed that neuroplasticity of the visual cortex or higher cortical areas may be the underlying mechanism of action.
Interestingly, a retrospective study on factors affecting recovery or restoration of neurological function reported that prolonged mental stress—which may be both a consequence and potential cause of neural inactivation—may influence outcomes.13 The study authors hypothesized that stress-prone personalities traits (i.e., neuroticism, greater conscientiousness) would more likely suffer from vascular dysregulation and would therefore benefit most from alternating current stimulation (ACS) therapy, which improves blood flow. However, their correlations suggested that stress-prone personalities recovered less from ACS and those with physiological signs of vascular dysregulation recovered more. While the cause-and-effect relationship between stress and neurological recovery is still unclear, the paper suggested that psychosocial factors and vascular dysregulation likely contribute to the “highly variable” outcomes of patients in low-vision therapy. Personalized care and therapy plans may play a role in future visual and neurological rehabilitation efforts.
Accessing Rehabilitation
Despite the positive reported outcomes of vision rehabilitation, very few glaucoma patients use visual rehabilitation services. In fact, a 2009 study reported that only 14 percent of patients receiving these services had a glaucoma diagnosis and only 10 percent of patients with low vision are referred for vision rehabillitation.14
There are a number of reasons why patients don’t receive visual rehabilitation. Barriers to care may include15,16
- lack of referral;
- ophthalmologists’ lack of awareness of these services (an AGS membership survey reported that only 22 percent of survey-takers were high referrers with knowledge of published low-vision services guidelines);17
- lack of appreciation of the benefits available from these services;
- lack of time in clinics to provide counseling;
- lack of functional issues reported by patients;
- lack of transportation to services; and
- lack of financial resources to purchase low-vision devices.
For patients, many of these issues create a vicious cycle in which their functional issues make it increasingly difficult to secure transportation or funds to attend rehabilitation appointments. Therefore, their vision continues to deteriorate and they become further disadvantaged.
AAO Model of Vision Rehabilitation
A comprehensive vision rehabilitation plan may cover reading (the top reason patients seek vision rehabilitation),18 daily living activities, safety and psychosocial well-being. The Academy’s model of vision rehabilitation outlined in their Vision Rehabilitation Preferred Practice Pattern includes three levels:
- Level 1: Recognizing and responding to low vision. The first level depends on ophthalmologists recognizing patients with low vision and responding with education, counseling and/or referral to vision rehabilitation services.
- Level 2: Initiating clinician services. The second level includes services provided by a clinician specializing in vision rehabilitation.
- Level 3: Involving a multidisciplinary team. The third level’s multidisciplinary team may include, but isn’t limited to, clinicians, occupational therapists, social workers, psychologists, orientation and mobility trainers, community support groups, aging services, and transportation services.
Evaluating Patients
Visual acuity, visual fields and contrast sensitivity are the main components to evaluate when testing patients for low vision.
—Visual acuity testing. When testing visual acuity, it’s important to test with high contrast charts and bright lighting. Commonly used projection charts aren’t appropriate for this testing due to their low contrast and presentation in dark rooms. If using the ETDRS chart, bring it closer to the patient. The chart should be at a distance where at least the top line of letters can be seen by the patient.19
Watch the patient’s head posture and eye movements as visual acuity is tested. A head turn may indicate that a patient has scotoma or is using an eccentric viewing location. Eccentric viewing is common among patients with central vision loss. Unlike foveal fixation, eccentric fixation occurs on the functioning peripheral retina, that is, the preferred retinal locus. Eccentric peripheral retinal loci are associated with lower visual acuity and less stable fixation, but this doesn’t necessarily mean that patients with foveal fixation have better acuity. Foveal fixation may also be impaired and/or unstable. As patients shift fixation, measured visual acuity may vary. We can’t assume that decreased visual acuity or unstable fixation means a patient is using eccentric peripheral retinal loci.20
 |
|
Figure 2. The Vistech contrast test uses sine-wave and bar patterns to assess vision worse than 20/40. |
Refracting patients with low vision is different than refracting those with normal visual potential. Retinoscopy and trial frames are good options. One may consider using full-aperture trial lenses since these allow the eccentric fixator to move the head or eyes as needed. Confirm the patient’s refractive error after retinoscopy using fogging or cross-cylinder.21
—Visual field testing. When evaluating the patient’s remaining visual field, pay attention to the location, size, shape, density and number of scotoma(s).
The Minnesota Low-Vision Reading (MNREAD) Chart is useful for determining the relationship of a scotoma to fixation in patients with a foveal-sparing scotoma (Figure 1). This text-based chart, available in multiple languages, assesses reading acuity (the smallest readable print); maximum reading speed (reading speed when performance isn’t limited by print size); and critical print size (smallest readable print at maximum reading speed).22 Print size decreases by 0.1 log unit steps, from 1.3 logMAR (Snellen equivalent 20/400 at 40 cm) to -0.5 logMAR (Snellen equivalent 20/6).
A patient with a scotoma to the right of fixation will find the next words in the chart obscured; with a scotoma to the left of fixation, one will have difficulty reading the beginning of the line. Scotomas above and/or below the peripheral retinal loci affect reading of the columns of numbers.
Another vision test for assessing scotomas called SKread is based on random word sequences. The unpredictable word and letter sequences make reading performance more dependent on eyesight rather than reading skill or educational level.23
—Contrast sensitivity testing. Contrast sensitivity is important for many daily tasks such as recognizing faces, objects, where steps begin and end, and for night driving. Two tests that are useful for assessing contrast sensitivity are the Vistech contrast test (Figure 2), which uses sine-wave and bar patterns to assess vision worse than 20/40, and the Pelli-Robson contrast sensitivity chart (Figure 3), which uses letters of the same size but with decreasing contrast.
 Figure 3. The Pelli-Robson chart uses letters of the same size in decreasing contrast to evaluate contrast sensitivity. |
—Discussion of needs, limitations and goals. Be sure to talk to patients about their current limitations and needs, such as how their decreased vision affects their daily activities. Identifying low-vision patients and gauging their needs may be supplemented by administering the NEI VFQ-9 questionnaire, an abbreviated version of the NEI’s 25-item Vision Function Questionnaire (NEI VFQ-25). Other questionnaires for screening patients for functional complaints, quality of life, and activities of daily living include the Activities of Daily Vision Scale (ADVS), Visual Function Index (VF-14), Visual Activities Questionnaire (VAQ) and Glaucoma Quality of Life (GQL-15).
Having conversations about realistic expectations is also important before embarking on a rehabilitation path. Though vision rehabilitation can improve many aspects of patients’ lives, it isn’t a magic bullet and doesn’t restore their vision to pre-disease states. Focusing on management strategies to optimize remaining vision is key.
—Ocular/systemic disease evaluation. Evaluate any co-existing ocular diseases that patients may have. About two-thirds of patients with low vision have systemic diseases. Pay particular attention to diabetes, arthritis, and any neurological disorders such as tremors, paralysis or weakness.
—Inquire about visual hallucinations. Charles Bonnet Syndrome, which occurs in about a third of patients with some degree of vision loss, produces visual (but not auditory or other sensory) hallucinations.24,25 Patients may see patterns, detailed images of people, places or events, or even imaginary creatures. CBS is attributed to a cortical release phenomenon resulting from lack of afferent visual information. In the absence of this visual data, the brain fills in the gaps with made-up images or recalls images from memory. These visual hallucinations usually stop after 12 months in about a quarter of patients. Managing strategies for coping with CBS, such as talking about the hallucinations with a trusted individual, resting, moving the eyes or looking away from the hallucination, or changing their environment can help the hallucinations to decrease or stop after a few years. Individuals with CBS are aware that what they’re seeing isn’t real.
Low-vision Interventions
There are numerous low-vision interventions available to patients. I recommend having examples of low-vision tools in the office to educate patients and normalize the devices and their use. Here are some tools to be aware of:
—Optical devices. Optical devices such as spectacles, magnifiers and telescopes are a good starting point for rehabilitation. High add-power glasses and high-plus reading glasses offer hands-free magnification and a large field of vision. These are best for short working distances and require good lighting. Readers with +4 D add are available over the counter. Readers greater than +4 D add require base in prisms to assist convergence and relax accommodation. Prism strength is 2 D more than the add.
To quickly estimate the needed add power, take the inverse of the patient’s visual acuity (Kestenbaum Rule). For example, a patient with a 20/200 visual acuity would require 200/20 or 10 D of add. This rule doesn’t factor in the effects of scotomas or decreased contrast sensitivity, however.
Handheld magnifiers can help patients perform short-term tasks such as browsing a menu. For longer tasks such as reading or near work, or for patients with tremors, stand magnifiers can help. Low-powered (+5 to +12 D) magnifiers are more commonly used due to comfort.
For higher magnification at a greater working distance than reading glasses, patients can opt for telemicroscopes, also called loupes. These are spectacle-mounted, binocular or monocular telescopes for near or distance tasks. They allow for a greater working distance than high-add reading glasses, but the visual field they offer is narrow, and depth of field is also reduced. These devices are useful for stationary distance viewing activities such as watching TV shows or sports. High minus lenses and reverse telescopes may be used for patients with severe peripheral field loss who retain good central vision. These devices decrease image sizes (with an accompanying decrease in acuity). This enables more visual information to fit within the patient’s small visual field.
Driving is an important issue to patients with vision problems related to glaucoma. Some states require special visual field testing (e.g., HVF 60-2) that patients may need to submit to their state division of motor vehicles. Also, states may allow bioptic driving, which allows for a small telescopic lens affixed to the standard lens on the better-seeing eye. A qualified driver with low vision may drive during daylight hours using a bioptic lens system. Availability of support for clinicians to gather required testing, complete forms, and services for fitting standard and low-vision optical devices are increasingly important in clinical care.
—Electronic devices. Smart magnifiers and video magnifiers, including computers, tablets, smartphones; and handheld, desk-mounted or head-mounted devices provide adjustable magnification and enhanced or reversed contrast without the peripheral distortion seen with glasses. Many feature text-to-speech conversion, using optical character recognition, or voice commands and voice outputs/screen readers. Many of these features can be activated in smartphones’ accessibility settings. There are also several smartphone apps for visually impaired individuals. Electronic devices in general tend to be costly.
—Non-optical aids. Other options that can help individuals with low vision include direct task lighting such as gooseneck lamps or pocket flashlights; large-print or high-contrast reading material; sensory substitution such as tactile feedback (e.g., felt-tipped pens vs. ballpoint pens); and typoscopes, which are inexpensive cards that allow a patient to focus on one line of text at a time and help to filter out excess information and reduce glare.
Environmental modifications may also be employed. Tripping hazards in the home can be reduced by marking steps or certain objects with high-contrast tape. Occupational therapy can assist patients with head- and eye-scanning strategies to increase environmental awareness and mobility. Some patients may opt for using a white cane to aid obstacle detection or signal to others that they’re visually impaired.
With so many options and often limited clinic time, it’s a good idea to have a handout to give to patients explaining low vision, rehabilitation and intervention options. The Academy’s handout on low vision for patients can be found at aao.org/low-vision-and-vision-rehab (scroll to “Materials for Patients with Low Vision”). This page also contains a link to the Vision Rehabilitation Preferred Practice Pattern guidelines.
Glaucoma is a difficult disease to manage, and the visual changes that accompany it are often frightening and distressing for patients. We must be aware of how this disease impacts patients’ functional daily living and be ready to recommend vision rehabilitation. Low vision management in glaucoma requires a multidisciplinary effort but the results can make a major difference for patients.
Dr. Singh is a professor of ophthalmology and chief of the Glaucoma Division at Stanford University School of Medicine. Dr. Netland is Vernah Scott Moyston Professor and Chair at the University of Virginia in Charlottesville.
Dr. Salim is a professor of ophthalmology, the vice chair for clinical and academic affairs and the director of the glaucoma service at The New England Eye Center at Tufts University School of Medicine. She has no related financial disclosures.
1. Chan T, Friedman DS, Bradley C, and Massof R. Estimates of incidence and prevalence of visual impairment, low vision, and blindness in the United States. JAMA Ophthalmol 2018;136:1:12-19.
2. Noellett CL, Bray N, Bunce C, et al. Depression in Visual Impairment Trial (DEPVIT): A randomized clinical trial of depression treatments in people with low vision. Invest Ophthalmol Vis Sci 2016;57:10:4247-4254.
3. Hinds A, Sinclair A, Park J, et al. Impact of an interdisciplinary low vision service on the quality of life of low vision patients. Br J Ophthalmol 2003;87:11:1391-1396.
4. McCarty CA, Mukesh BN, Fu CL, et al. Risk factors for age-related maculopathy: The Visual Impairment Project. Br J Ophthalmol 2001;119:10:1455-1462.
5. Klein R, Klein BEK, Jensen SC, et al. The five-year incidence and progression of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmology 1997;104:1:7-21.
6. Ramulu P. Glaucoma and disability: Which tasks are affected, and at what stage of disease? Curr Opin Ophthalmol 2009;20:2:92.
7. Mills RP, et al. Correlation of visiual field with quality-of-life measures at diagnosis in the Collaborative Initial Glaucoma Treatment Study (CIGTS). J Glaucoma 2001;10:3:192-198.
8. Goldstein JE, Jackson M, Fox SM, et al. Clinically meaningful rehabilitation outcomes of low vision patients served by outpatient clinical centers. JAMA Ophthalmol 2015;133:7:762-769.
9. Lamoureux EL, Pallant JF, Pesudovs K, et al. The effectiveness of low-vision rehabilitation on participation in daily living and quality of life. Invest Ophthalmol Vis Sci 2007;48:4:1476-1482.
10. Livengood HM, Baker NA. The role of occupational therapy in vision rehabilitation of individuals with glaucoma. Disabil Rehabil 2015;37:13:1202-1208.
11. Kaleem MA, Rajjoub R, Schiefer C, et al. Characteristics of glaucoma patients attending a vision rehabilitation service. Ophthalmol Glaucoma 2021;4:6:638-645.
12. Sabel BA, Gudlin J. Vision restoration training for glaucoma: A randomized clinical trial. JAMA Ophthalmol 2014;132:4:381-389.
13. Sabel BA, Wang J, Fähse S, et al. Personality and stress influence vison restoration and recovery in glaucoma and optic neuropathy following alternating current stimulation: Implications for personalized neuromodulation and rehabilitation. EPMA J 2020;11:2:177-196.
14. Owsley C, Mcgwin G, Lee P, et al. Characteristics of low-vision rehabilitation services in the United States. Arch Ophthalmol 2009;127:5:681-689.
15. Deemer et al. Eye 2022. In Press.
16. Overbury O, Wittich W. Barriers to low vision rehabilitation: The Montreal Barriers Study. Invest Ophthalmol Vis Sci 2011;52:8933-8938.
17. Kaleem MA, West SK, Im LT, et al. Referral to low vision services for glaucoma patients: Referral patterns and characteristics of those who refer. J Glaucoma 2017;26:e115-e120.
18. Aspinall PA, Johnson ZK, Azuara-Blanco A, et al. Evaluation of quality of life and priorities of patients with glaucoma. Invest Ophthalmol Vis Sci 2008;49:5:1907-1915.
19. Gilbert C and van Dijk K. When someone has low vision. Community Eye Health 2012;25:77:4-11.
20. Martin TD, MacKeben M, Schuchard RA, Fletcher DC. Foveal vs. eccentric PRL characteristics for fixation in low vision patients. ARVO Meeting Abstract, May 2008. Invest Ophthalmol Vis Sci 2008;49;13:4103.
21. Sahli E, Idil A. A common approach to low vision: Examination and rehabilitation of the patient with low vision. Turk J Ophthalmol 2019;49:2:89-98.
22. MNREAD Acuity Charts. https://legge.psych.umn.edu/mnread-acuity-charts. Accessed November 22, 2022.
23. MacKeben M, Nair UKW, Walker LL. Random word recognition chart helps scotoma assessment in low vision. Optom Vis Sci 2015;92:4:421-428.
24. O’Brien J, Taylor JP, Ballard C, et al. Visual hallucinations in neurological and ophthalmic disease: Pathophysiology and management. J Neurol Neurosurg Psychiatry 2020;91:5:512-519.
25. Cox TM, ffytche DH. Negative outcome Charles Bonnet Syndrome. Br J Ophthalmol 2014;98:91236-1239.





