Of all the tools in a glaucoma surgeon’s armamentarium, endoscopic cylcophotocoagulation—in which fiber optics allow the surgeon to partially coagulate ciliary process tissue from inside the eye, reducing the production of aqueous—may be one of the least often used. I suspect that most surgeons think of it as the final option in the toolbox: When everything else has failed, you resort to ECP to try to save the eye from a really high pressure that you haven’t been able to control with a tube or trabeculectomy. In addition, my impression is that in most places, when a patient needs an ECP for refractory glaucoma, the doctor usually sends him to a vitreoretinal surgeon who goes in via the pars plana to do the procedure.
I began performing ECP in 2004 when I trained at the University of California San Francisco. In 2007 I did my fellowship with Ike Ahmed, MD, who introduced me to the concept of endoscopic cycloplasty (more on that in a minute). I’ve been performing ECP ever since, and I’ve found it to be a very useful tool for many patients with glaucoma or ocular hypertension.
Here, I’d like to explain how I use ECP and why I believe many more patients could be benefiting from it.
The Advantages of ECP
ECP is a bit of a unique animal, in that almost all other combined cataract/glaucoma procedures are mainly aimed at increasing the outflow of aqueous. ECP acts by reducing the creation of aqueous—turning down the tap a little bit, so to speak.
One of the strongest points in favor of ECP is its low-risk profile.
Previously, the comparison would have been to tubes or trabeculectomies, which of course have a much higher risk profile. With trabeculectomy, for example, you have a lifetime risk of infections, bleb failure and other potentially visually devastating complications. Today, however, the landscape of glaucoma surgery is changing with the advent of micro-invasive glaucoma surgery devices like the iStent or Trabectome, which are making glaucoma surgery a lot safer. But even compared to these options, I see ECP as being on the low end of the risk spectrum. Devices, while overall fairly safe, have the potential to dislocate inside the eye, and they are foreign bodies that may incite inflammatory responses or result in hyphema or angle bleeding. Furthermore, those procedures are more technically challenging than ECP is to perform. So, I still believe that ECP is one of the lowest-risk options available.
| ||||||
Another advantage of the ECP procedure is that the visualization of the ciliary body via the fiber optic viewer occasionally reveals problems that were otherwise invisible to the surgeon. We’ve treated some patients who had very narrow angles and discovered large cysts in the ciliary body or iris. Sometimes these can be seen preoperatively on gonioscopy or via ultrasound biomicroscopy, but not always. That discovery can give you a window into the reason for certain pathologies.
ECP is also titratable; it allows the surgeon to adjust the size of the arc being targeted with the laser based on the patient’s status. I usually “paint” about 270 degrees, just because that’s the approximate arc I can comfortably reach through a single temporal incision. But if a patient has refractory glaucoma you can easily make a second incision allowing you to cover 360 degrees. (On the other hand, if you need that much ablation you may be looking at advanced glaucoma that might be better addressed with a different procedure.)
ECP: Opening Narrow Angles
Perhaps the most useful role for ECP in cataract surgery when glaucoma is a concern is in patients who have narrow angles. Most surgeons think of ECP as a means to reduce IOP by decreasing aqueous production, and it certainly is useful in that capacity. However, ECP also allows us to manipulate the position and size of the ciliary body and processes. That means we can open up the angle in patients who have narrow angles at the time of cataract surgery. My colleague Dr. Ahmed coined the term “endoscopic cycloplasty” (abbreviated ECPL) to describe this use of ECP.
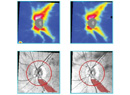
The technique is fairly straight-forward. Wherever the laser strikes, the tissue is coagulated, and the remaining tissue shrinks towards the coagulated area. So in a narrow-angle patient, you aim the laser at the posterior end of the process. After you fire the laser, the process shrinks downward, opening the angle. (See images, p. 82.) Obviously, you would not want to start at the top of the process, which would have the opposite effect.
I’ve seen some very dramatic examples of this treatment’s effectiveness in patients whose angles were almost appositional; you could hardly see the trabecular meshwork at all before surgery. After surgery with ECP, the angle is typically wide open, Shaffer grade 4. This is especially useful in patients who have plateau iris, where the ciliary processes are riding very high up. (Cataract surgery by itself may help these patients a little, but some patients with plateau iris can still end up with very narrow angles.)
| ||||||||
Of course, patients sometimes have combined- or mixed-mechanism glaucoma, where they do have narrow angles, but that alone isn’t sufficient to account for the amount of disc disease or visual field loss. For example, a patient may have grade-2 angles with no visible synechiae or evidence of intermittent or chronic angle closure, yet he has a large field defect or a notch on the nerve and his pressure is in the mid-20s. Clearly, this patient also has some trabecular meshwork dysfunction. Of course, you can still use ECP in those cases, but I’m a little less optimistic that ECP alone will get those patients to your target pressure, depending on what pressure you’re aiming for.
Addressing Surgeon Reluctance
Given the usefulness of the procedure, why are so many surgeons reluctant to use it? Part of the reason may be a fear of hypotony, which many surgeons associate with cycloablation procedures. This is ironic, because I’ve performed ECP for nine years, and I have yet to see a patient—cataract or otherwise—who had hypotony issues afterward. In contrast, I’ve seen a number of cases where we did cataract surgery with a tube shunt or trabeculectomy and the patient had hypotony for weeks while we waited for the pressure to increase a little bit.
One reason that ECP doesn’t lead to hypotony is that there appears to be little danger of destroying all of the aqueous-producing epithelium, even if you treat 360 degrees. The ciliary body has a large surface area because of all the nooks and crannies and the cracks between each ciliary process. In the usual ECP treatment, the laser simply doesn’t treat all of the tissue in the area at which it’s aimed.
The fear that ECP will lead to hypotony may be the result of people automatically thinking of transscleral cyclophotocoagulation, which produces an entirely different degree of tissue destruction from ECP. With TCP, where the laser is targeted from outside the eye, the energy can affect a much broader tissue area. Many histological studies have demonstrated that while TCP can do massive damage, ECP doesn’t destroy underlying tissue structure. TCP is a much more invasive process, and—unlike ECP—definitely brings with it a risk of hypotony.
Unfortunately, it only takes one case producing unwanted results to cause a surgeon to avoid a procedure. I’ve noted, for example, that
a lot of surgeons who routinely use
intracameral antibiotics have experienced a case of endophthalmitis that didn’t go well. Similarly, I think that when people have seen tissue destruction or hypotony from TCP, they become afraid to resort to cycloablation, even though those concerns do not directly apply to ECP.
Another likely reason for less-frequent use of ECP is the cost of the instrument. To many surgeons, it’s a tool of last resort. And although it’s especially helpful for treating patients with narrow angles, practice demographics may limit the number of patients you see who have narrow angles. If you believe you’ll only use it once a year, it’s understandable that you might hesitate to spend the money to purchase the instrument.
When ECP Isn’t Ideal
One of ECP’s limitations is its inability to produce a very low target pressure. Typically, ECP only produces a pressure in the mid-teens (a criticism often leveled at other minimally invasive glaucoma procedures as well). That’s because of episcleral venous pressure, which limits how low you can get the pressure using these approaches. Of course, this also is the argument as to why these procedures are safer; the risk of hypotony with a Schlemm’s canal device is extremely low, and the same is true for ECP.
Nevertheless, if your target pressure is very low, ECP is probably not your procedure of choice. If a patient has a severe hemifield cut and you want his pressure to be 10 mmHg, you probably would want to use a different treatment. On the other hand, if the patient has a very early arcuate scotoma and very mild mean deviation and your target pressure is in the mid-teens, I’d say ECP would be an excellent option to choose.
Another thing we’ve noted, although we don’t have clinical data to support this yet, is that ECP is not very efficacious in pseudoexfoliation patients. Even when we do the ECP intraoperatively, the ciliary processes don’t seem to take up the laser energy as well. We can’t say for certain what the reason is, but if you look at the processes with an endoscope during surgery, you can see the pseudoexfoliation material covering the zonules, and the ciliary processes look more white. So it could be a matter of less pigment being present, or possibly the result of the dandruff-like pseudoexfoliation material blocking the uptake of the laser. You typically have to turn the laser power up higher with these patients, and even so, the processes don’t seem to respond quite as dramatically during surgery.
This is not to say that a patient with pseudoexfoliation isn’t a candidate for ECP. I would still try ECP as a first-line therapy if the patient had pseudoexfoliation but has a narrow angle and a mild degree of glaucoma. In that situation, ECP might indeed help to open the angle and get the patient to a lower pressure, allowing him to avoid a more invasive procedure like a trabeculectomy down the road.
I also don’t use ECP in patients who have a history of uveitis or uveitic glaucoma, primarily for two reasons. First, ECP will stir up more inflammation postoperatively—at least in the early postop period—and in these patients, minimal inflammation is desirable. Many of them will have difficulty getting their uveitis controlled even if cataract surgery is performed without ECP. Second, although uveitic patients can have a high intraocular pressure, they are also prone to hyposecretion where the ciliary body can shut down and produce very little aqueous, leading to hypotony.
An Option with Promise
Despite its lack of widespread use, ECP has potential as a tool for addressing certain types of glaucoma—in particular, glaucoma attributable to a narrow angle. Given the difficulty and importance of addressing this disease, I think it’s a tool more surgeons should consider adding to their armamentariums. REVIEW
Dr. Tam is in private practice in the Toronto area. He has no financial interest in any of the technologies discussed above.