When we think of donor tissue in ophthalmology, corneal transplantation is what leaps to mind. And with good reason—it is now the most frequently performed of all human transplant procedures. In the past 50 years, more than 1 million people have had their vision restored through corneal transplant. The success of this procedure is due not only to the generous donors and skilled surgeons but also to the successful development of eye banks across the United States. These nonprofit organizations deserve a tremendous amount of credit for making corneal transplantation safe and widely available.
Less well known, perhaps, is the growing importance of eye banking and ocular tissue in ophthalmic research. Tissue studies aren’t new, of course—some of us have relied for decades on eye bank tissue for our research. But new models in eye banking are making it possible to get fresher, better-characterized tissue. At the same time, new molecular and genetic research techniques are expanding what can be learned from tissue samples. In combination, these trends bring us to the cusp of great progress in understanding and intervening in complex disease processes like glaucoma, age-related macular degeneration and diabetic eye disease.
Limitations of Animal Models
Animal models have certainly been of tremendous value in ophthalmic research. Rabbits, mice and rats have provided us with a plentiful and inexpensive resource for studying diseases and clinical interventions.
One of the greatest advantages of animal models is that they allow for very tight control of genetic and environmental factors. In my lab, for example, we use “humanized” mice that carry cloned genes responsible for certain proteins normally found in the human crystalline lens (See Figure 1). Such mice are genetically homogeneous and are raised in the same environment, so we don’t have to worry about the confounding effects of variations in genetic makeup, diet, sunlight and environmental exposures, medication history, or other factors that one cannot control in a human population.
| ||||||
Physiologically, there are species-specific differences in key ocular structures such as the trabecular meshwork and optic nerve head. Rodents have no macula, and their corneas are thinner than human’s, while their lenses almost completely fill the globe.
Experimental animals have short life spans, making it very difficult to study degenerative, age-related diseases like cataract and AMD. In fact, we have no adequate animal models at all for AMD, according to Gerald Chader, PhD, chief scientific officer at the Doheny Retina Institute and professor of ophthalmology at the University of Southern California in Los Angeles. “It is very difficult to induce drusen, and also difficult to produce neovascularization that closely mimics human pathology in any animal eye,” he notes. “So we can only approximate the classic signs of either wet or dry AMD in animals.”
Even in good experimental models—such as our humanized mouse lenses—we are forced to create an artificial system that cannot be expected to reproduce many of the molecular and genetic control points that could be important factors in human ocular diseases. That is why, despite being very good at studying the pathogenesis of disease and preventing blindness in experimental animals, it is still challenging to translate therapeutic strategies into humans.
Laboratory research on donor tissue is the key intermediate step between experimental animal research and human clinical trials. By expanding the experimental work that is done on human eyes, we can begin to answer some of the big questions in ophthalmology and, at the same time, better guide and shape the therapies that will eventually be brought to the clinic.
Lens
Preventing PCO and diabetic cataract. In my laboratory at the University of Colorado-Denver, one area of focus is secondary cataract or posterior capsular opacification that occurs after phacoemulsification. In a significant proportion of cataract patients, residual lens epithelial cells proliferate and cause a secondary opacity, requiring another surgical procedure.
|
Another major research focus in our lab is the molecular mechanism linking diabetes to early-onset cataract and retinopathy. In diabetes, chronic hyperglycemia accelerates a metabolic pathway that is not usually activated. With the increasing incidence of diabetes, diabetic eye disease has the potential to affect many people worldwide. Our humanized mouse models have been tremendously helpful as a drug discovery platform in our search for inhibitors of diabetic eye disease. However, the true validation of our work to date must rely on testing carried out with human-derived cells and tissues.
Glaucoma
Pathways for intervention. Researchers at the University of North Texas Health Science Center (NTHSC) are investigating molecular pathways implicated in another important ophthalmic disease: glaucoma.
Abbot F. Clark, PhD, FARVO, professor of cell biology and anatomy at NTHSC and the director of the North Texas Eye Research Institute in Ft. Worth, has discovered several pathogenic pathways in glaucoma using human tissue models. By harvesting ribonucleic acid from cultured trabecular meshwork and optic nerve cells and from the donor tissue itself, he and his colleagues have been able to compare gene expression in normal eyes to those with glaucoma. “The RNA must be obtained from very fresh tissue,” says Dr. Clark, adding that after partial processing of the tissue, much of the analysis can be done later. He hopes soon to be able to begin culturing and analyzing retinal ganglion cells, as well.
One example of the pathways identified and validated by his team involves transforming growth factor (TGF) β2. “In healthy eyes, bone morphogenetic proteins (BMPs) modify the pro-fibrotic signaling of TGFβ2 to help maintain trabecular meshwork function, but this delicate balance gets upset in glaucoma,” Dr. Clark says. “We have found significantly elevated expression of gremlin mRNA, a BMP inhibitor, in both the TM and optic nerve head cells cultured from eyes with glaucoma, increasing IOP.”1-3 (See Figure 3). Other signaling pathways the team has identified also decrease outflow facility and increase IOP.
“It certainly appears that there are a number of ways to perturb the system and contribute to the disease process in glaucoma, but this also means there are multiple potential therapeutic targets to intervene in that process,” he says.
Cornea
From device performance to drug penetration. Eye banking, of course, was built around the need for donor corneas. But corneal tissue is also used extensively for research purposes, some of which supports transplantation—for example, the development of eye bank storage protocols and new femtosecond laser-assisted keratoplasty procedures. But there are many other important areas of inquiry, according to Henry F. Edelhauser, PhD, Emory University professor of ophthalmology, whose involvement in corneal tissue research dates back to the development of surgical irrigating solutions in the 1970s.
That work began in rabbits, but the animal studies weren’t enough. “The corneas of young animals are able to withstand insult and stress better than older human corneas,” Dr. Edelhauser says, “so we needed to verify that the intraocular irrigating solutions could protect the corneal endothelium in 70- or 80-year-old cataract patients.” Fortunately for modern cataract surgery, they proved adequate to the task.
Since then, he and others in the field of corneal research have provided valuable insight into the impact of other drugs and devices on the corneal endothelium, corneal toxicity and the wound-healing response in human corneas after corneal refractive surgery. Dr. Edelhauser credits Emory’s large collection of more than 550 post-LASIK corneas with providing essential information about wound healing and the tensile strength in these LASIK corneas.4-6 “This is another area where age matters,” he says. “The young tissue from rabbit corneas heals differently than the LASIK flap in human patients.”
Drug penetration studies typically begin in animals, allowing companies to screen lots of drug candidates and concentrations to determine how they diffuse through and across the cornea, where they bind and whether there is potential toxicity. “But then, the use of human corneal tissue plays a very important role in speeding up the experimental process and making it safer to move to human clinical trials,” says Dr. Edelhauser.
His latest research is proof of the value of human tissue in drug studies.Dr. Edelhauser’s team is in the process of evaluating the distribution of various drugs and antibodies that can potentially be used in the treatment of AMD following microneedle injection into the suprachoridal space of the human eye. (Patel SR, et al. IOVS 2011;52:ARVO E-Abstract 3255)
Retina
A wide-open field for AMD research. “Retinal research is among the most difficult to conduct in human tissue because the delicate retina is so sensitive to postmortem degenerative changes,” says Dr. Chader. “Yet it is also the area of greatest opportunity because our animal models for retinal disease are so poor.”
|
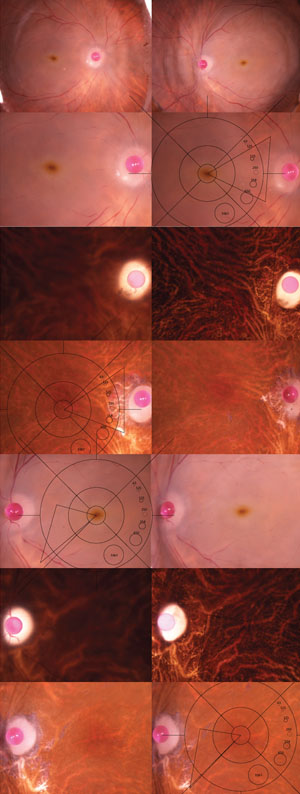
“For detailed biochemistry and gene expression studies, we’re primarily interested in tissue that can be obtained less than six hours post-mortem,” Dr. Olsen says. To deal with this time-dependent challenge, he has trained technicians at the Lions Eye Institute for Transplant and Research (LEITR) in Tampa, Fla., to process the tissue and obtain high-resolution, digital, color stereo fundus photographs of the eyes immediately upon procurement (See Figure 2). The fundus photos are uploaded to a shared database for grading by Dr. Olsen’s team and the tissue is flash-frozen for subsequent molecular analysis.
“We are really fortunate to be able to take what we learn from genomic and proteomic studies using eye bank tissue and combine this data with longitudinal data from the living AREDS population,” Dr. Olsen says. “The common link between the two is the grading system. To date, the team has graded nearly 400 eyes, including eyes from across the recently expanded nine-point AREDS scale,9 as well as age-matched controls. We’ve used this tissue in Minnesota in conjunction with Dr. Deborah Ferrington’s lab to examine proteins in the neurosensory retina and the retinal pigment epithelium at different stages of AMD,” Dr. Olsen says. Initially, he expected to find significant protein changes at the end stage of the disease. “But we actually found the highest levels of abnormal proteins in eyes in that key transition from AREDS 2 to AREDS 3—the exact stage when a clinician should recommend AREDS supplements,” he says.
“The correlation biochemically is remarkably consistent with the antioxidant data seen clinically in AREDS,” he says. However, Dr. Olsen indicates that his work supports the involvement of multiple biochemical pathways. “In fact, what we call AMD probably has many different causes that result in a final common phenotypic expression,” he says. “The ability to compare genetic variants or single nucleotide polymorphisms (SNPs) from tissue samples at earlier disease stages will continue to lead us to understand multiple other pathways,” says Dr. Olsen. For example, he is now investigating gene regulation of mitochondrial damage and injury that influence cell death.10
It is worth reiterating that, because there is no good animal model for macular degeneration, Dr. Olsen’s work—and the important lessons about the etiology and pathology of AMD that may come from it—are entirely dependent on access to human tissue from eyes with the disease.
Eventually, Dr. Olsen hopes the eye bank will be able to retain and distribute his graded tissue samples for use by other researchers, too. “If we can centralize the data into a bioinformatics network, it could enable researchers all over the country to correlate their findings into an intelligent and interactive network and perhaps arrive at more conclusive pathways for treatment that no one team could see independently,” he says.
When he presents on this topic at scientific meetings, other researchers almost immediately see the value in the tissue samples. But adding enough samples to the data bank takes money. To make his dream of a bioinformatics network a reality, he is asking that more researchers consider this multicentered collaboration and add the eye bank tissue into their research grants just as they would budget for transgenic mice or other materials.
Eye Banks in Research
“Human tissue research has always been possible, but in the past it wasn’t very convenient,” says Dr. Edelhauser. That is changing as eye banks begin to appreciate the enhanced role they might play in supporting research.
In general, only university-based or very large eye banks have the volume and staff resources to consistently provide tissue within the tight window of time and the parameters needed by investigators. They must be able, in most cases, to obtain whole globes (rather than just corneas with scleral rims) and begin to dissect and process them immediately.
The Lion’s Eye Institute for Transplant and Research is uniquely positioned to support the kinds of research discussed in this article. As the world’s first combined eye bank and ocular research facility, it is far more research-focused than a typical eye bank. LEITR allocates about half the donor tissue it collects for research purposes and takes extra steps to characterize the tissue according to the donor’s clinical and familial medical history. Tissue can be available within as little as four to six hours after death. To take advantage of this, researchers can use the facility’s on-site sleeping quarters and high-technology laboratories for rapid and round-the-clock access to tissue samples as they become available.
The Tampa facility draws from a large population of people with high rates of age-related ocular diseases, so it can quickly provide researchers with multiple pairs of eyes meeting its criteria. “This really changes how we can think about designing retina studies,” says Dr. Chader. “Researchers can be more confident that they will be able to obtain a sufficient number of eyes in a time frame that allows them to begin work while the sensitive retinal tissue is still viable.”
|
Maximizing the Gift
The studies I’ve described here are in most cases still at the basic science stage—years away from products that will be used to treat patients clinically. But their potential impact on costly, sight-threatening diseases like AMD, glaucoma and diabetic eye disease is tremendous.
There is an ongoing need, however, for donor tissue. Ophthalmologists are in a unique position to begin educating patients and their families about the importance of tissue donation. People with AMD or glaucoma are often surprised to learn that they may be eligible to donate ocular tissue—and they also are often strongly motivated to support research that may prevent their children or grandchildren from suffering from the same problems.
“As physicians, we can set an example by showing our families, patients and colleagues the importance of donation,” says Richard L. Lindstrom, MD, founding partner of Minnesota Eye Consultants.
One way to raise public awareness is simply to make local eye bank brochures available in the doctor’s office. Cards listing resources for more information, such as the national
areyouadonor.org website, make it easy for people to learn more when they’re ready.
Other doctors raise the topic personally, sharing with patients the important news that ocular pathologies don’t exclude them from being eye donors.
When people are educated about the value of eye donation, they often welcome the opportunity to help. Their gift can benefit transplant recipients in the near term. And in the long term, it is an amazing resource for scientists who are engaged in solving the mysteries in ophthalmology, saving sight and bringing new therapies to clinical use. REVIEW
Dr. Petrash is vice chair for research in the department of ophthalmology, University of Colorado-Denver, past president of the Association for Research and Vision in Ophthalmology and a member of the LEITR Research Advisory Board. Contact him at
Mark.Petrash@ucdenver.edu. Drs. Chader, Clark and Edelhauser also serve on the LEITR Research Advisory Board. To learn more about LEITR, contact
info@LionsEyeInstitute.org or visit
LionsEyeInstitute.org.
1. Fleenor DL, Shepard AR, Hellberg PE, et al. TGFbeta2-induced changes in human trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci 2006;47:226-234.
2. Wordinger RJ, Fleenor DL, Hellberg PE, et al. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci 2007;48:1191-200.
3. Wang WH, McNatt LG, Pang IH, et al. Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure. J Clin Invest 2008;118(3):1056-64.
4. Fournie PR, Gordon GM, Dawson DG, et al. Correlation between epithelial ingrowth and basement membrane remodeling in human corneas after laser-assisted in situ keratomileusis. Arch Ophthalmol 2010;128:426-36.
5. Randleman JB, Dawson DG, Grossniklaus HE, et al. Depth-dependent cohesive tensile strength in human donor corneas: Implications for refractive surgery. J Refract Surg 2008;24:S85-9.
6. Dawson DG, Schmack I, Holley GP, et al. Interface fluid syndrome in human eye bank corneas after LASIK: Causes and pathogenesis. Ophthalmology 2007;114:1848-59.
7. Olsen TW, Feng X. The Minnesota Grading System of eye bank eyes for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45(12):4484-90.
8. Olsen TW. The Minnesota Grading System using fundus autofluorescence of eye bank eyes: A correlation to age-related macular degeneration (an AOS thesis). Trans Am Ophthalmol Soc 2008;106:383-401.
9. Davis MD, Gangnon RE, Lee LY, et al; Age-Related Eye Disease Study Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol 2005;123:1484-98.
10. Karunadharma PP, Nordgaard CL, Olsen TW, Ferrington DA. Mitochondrial DNA damage as a potential mechanism for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010 Nov;51(11):5470-9.