There’s no question that corneal cross-linking has been a miraculous treatment for problems like keratoconus and post-surgical ectasia. But it’s also clear that cross-linking’s earliest protocols have been hard on patients. Today, new approaches to generating cross-linking in corneal tissue are getting closer than ever to becoming real.
Here, surgeons provide an update on several of the most promising new cross-linking variations, including two promising epi-on approaches; miniaturization of the equipment; and triggering cross-linking via an eye drop. In addition, surgeons offer updates on how they’re using cross-linking to address difficult-to-treat infections, and what the outlook is for using cross-linking to treat small refractive errors.
Effective Epi-on: Coming Soon?
Every surgeon involved with corneal cross-linking is well aware that the traditional epi-off procedure is painful for the patient and requires extensive healing time. For that reason, finding an effective epi-on procedure has long been the Holy Grail of the field. Now, two of the systems in development could soon become a reality.
The EpiSmart (epithelium-on) system, from CXL Ophthalmics (Encinitas, Calif.) recently completed a Phase II study for the FDA.1 The prospective, randomized and controlled study involved 2,228 patients, 1,922 of whom had been diagnosed with keratoconus. The primary endpoint for the study was corrected distance visual acuity, with UCVA, Kmax and minimum corneal thickness as secondary endpoints. Seventy-one percent of patients underwent bilateral, simultaneous treatment (which was possible because there was no need to remove the epithelium).
Study findings included:
- At six and 12 months, keratoconus patients showed improved CDVA, UCVA and Kmax. Minimum corneal thickness was unchanged.
- At six and 12 months, keratoconus patients showed significant improvements in CDVA, UCVA and Kmax (p<0.001). These improvements were shown in a prior study2 to be stable two years after the treatment.
- Minimal corneal thickness wasn’t reduced at any point after treatment, confirming the results of prior publications.3
- No bandage contact lenses were applied and patients experienced only brief discomfort, returning to normal activities the next day.
- Only 195 subjects (8.7 percent) reported any adverse event related to the treatment and none of those reported was serious. The safety profile was similar to that of a dry-eye medication. No corneal infections were reported.
- Only six out of 2,228 patients (0.3 percent) developed corneal haze or opacity post-surgery. (Haze or opacity is reported in 64 percent of those treated with the current FDA-approved epi-off procedure.)4
Roy S. Rubinfeld, MD, MA, medical director at Re:Vision in Rockville, Maryland, and Fairfax, Virginia, and a clinical professor of ophthalmology at Georgetown University Medical Center in Washington, DC, is the inventor of the EpiSmart system. “In 2008, while spending time in Europe, I became interested in crosslinking, which was already available there,” he explains. “Right away I was taken with the challenge of finding a way to get adequate riboflavin into the corneal stroma without removing or disrupting the epithelium. I worked on the problem for several years, with input from many exceptionally smart, experienced individuals. By 2011 we’d developed and filed patents on three key innovations.
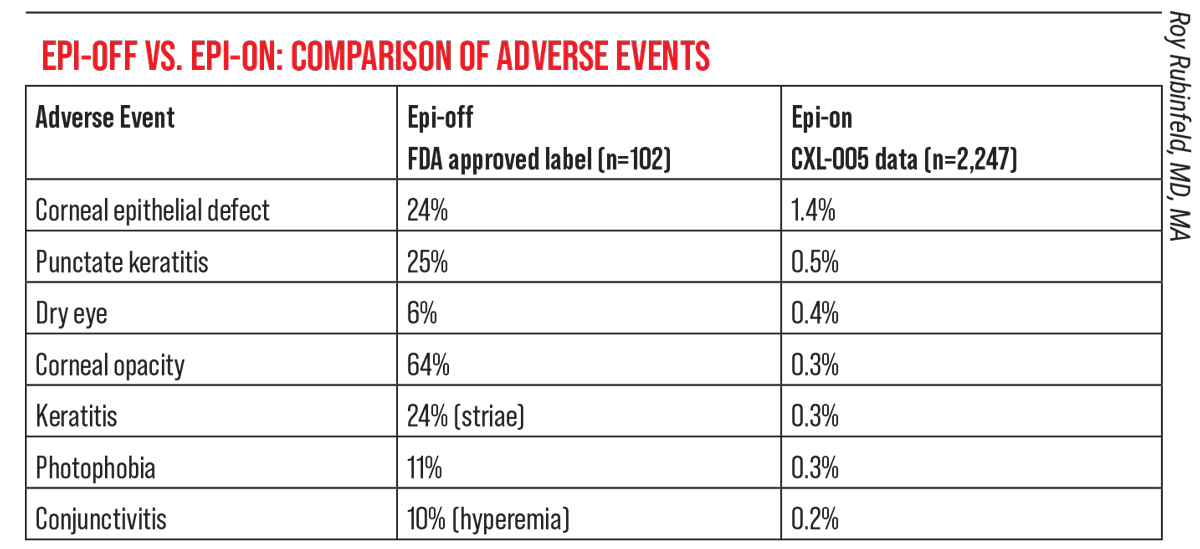 |
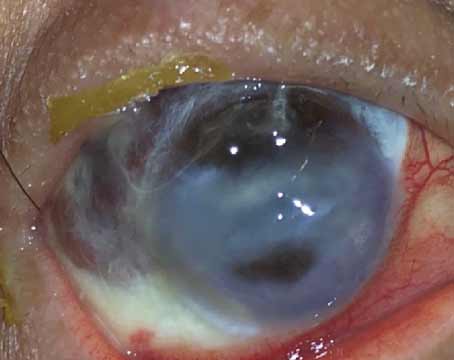
| Not having to remove the epithelium is an obvious boon for patients undergoing cross-linking, because removing the epithelium is painful and requires a lengthy recovery. However, leaving the epithelium undisturbed also reduces adverse events significantly. Click image to enlarge. |
“It was clear from our early testing that our new approach could be performed quickly, safely and in a bilateral, simultaneous procedure,” he says. “When I first started doing epi-off cross-linking, as part of an IRB-approved study, it was so painful and difficult for patients that my staff hated being part of it, and many patients didn’t even return to have the second eye done. With the new procedure, our patients are back at work or school the next morning. No bandage lenses are used, and we’ve gotten the UV treatment itself down to 20 minutes.”
Another epi-on system currently undergoing clinical trials is the iLink system from Glaukos/Avedro. The Epi-off version of the system is currently FDA-approved, but the epi-on version, which uses their proprietary Epioxa formulation, just completed its Phase III trial.
The company reports that Epioxa achieved its primary efficacy outcome in the Phase III trial by demonstrating a Kmax treatment effect of -1 D (p=0.0004) at six months, prospectively defined as least square mean Kmax change from baseline in treated subjects compared to those treated with placebo. In the treatment arm, Kmax improved by 0.2 D; the subjects treated with placebo experienced a worsening of 0.8 D.
The company also notes that 98 percent of the subjects randomized to placebo elected to cross over to the Epioxa treatment. Those switching to Epioxa showed an improvement of 0.3 D after six months of treatment. The majority of adverse events were mild and transient. No change in endothelial cell counts was observed.
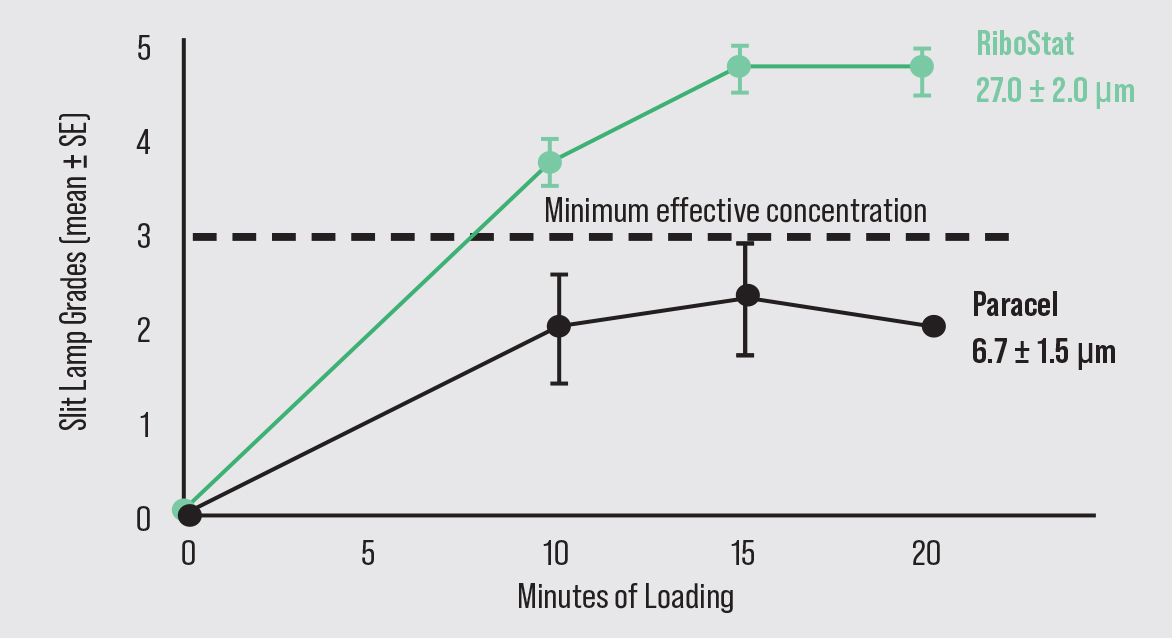 |
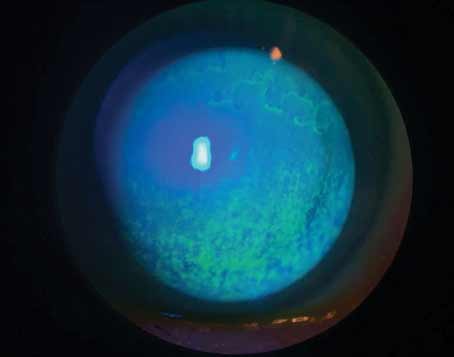
| A key challenge for creating epi-on cross-linking has been getting the necessary amount of riboflavin through the epithelium, without disrupting it, to make successful cross-linking possible. The EpiSmart system’s formula, RiboStat, has demonstrated in clinical trials that it can accomplish this. Photo: Roy Rubinfeld, MD, MA. Click image to enlarge. |
Epi-on: The Science
“To make epi-on crosslinking effective you need the correct amounts of the three key reagents—riboflavin, UV light and oxygen—in the stromal microenvironment at the same time,” Dr. Rubinfeld explains. “Until recently, no epi-on system has been able to adequately load the stroma without epithelial disruption or the use of iontophoresis.5 One key innovation in the EpiSmart system that enables epi-on riboflavin loading is the sodium iodide in our formulation.6 This increases epithelial permeability without disrupting the epithelium.
“Another stromal loading innovation is the patented disposable sponge applicators,2 which have unique shapes and physical and hydration characteristics,” he continues. “The first applicator is hydrated with BSS or anesthetic drops. This removes the lipids sitting on the surface of the cornea, so that some of the barrier to loading of the stroma is removed, but it doesn’t disrupt the epithelium. Then another sponge loaded with the Ribostat formula is placed on top of the cornea to act as a loading depot. When riboflavin is delivered in drops, the drops often roll right off the cornea, but with the loading sponge, that doesn’t happen.
“After loading, the surgeon looks at the cornea using the slit lamp to confirm that the green riboflavin is adequately and evenly distributed across the stroma,” he says. “This type of slit-lamp grading has been validated with lab tests such as HPLC and mass spectrometry.”7
Dr. Rubinfeld notes another positive effect of having sodium iodide in the formulation. “It helps prevent UV light from photodegrading the riboflavin into inactive materials that don’t assist in crosslinking,” he explains. “If any hydrogen peroxide is formed, for example, the sodium iodide turns it back into oxygen and water, fueling the cross-linking reaction and avoiding toxicity.8,9 Since oxygen is the rate-limiting reagent, this helps optimize oxygen availability in the stroma and avoids the highly variable blocking of UV transmission into the stroma, sometimes called the ‘sunscreen effect.’
 |
| The CXLens performs cross-linking via a contact lens placed on the eye, which the company says increases patient comfort and eliminates the need for eye tracking. |
“Also, you can’t get oxygen into the cornea when there’s riboflavin on the surface and the UV light is on,” he points out. “In that situation, the added riboflavin photodegrades and ‘burns up’ the oxygen at the surface. For that reason, no riboflavin drops are used during the UVA application in our protocol.”
Dr. Rubinfeld says another innovation involves the UV light. “Using a UV light that’s constantly on actually undercuts the photochemistry of the process,” he explains. “When the UV light is on, oxygen (the rate-limiting reagent) is being depleted, hypoxia is created and you end up with a toxic stew of hydrogen peroxide and other toxic molecules. Having the light turn on and off at the correct cycling time allows the oxygen to rediffuse through the cornea, replenishing oxygen while it’s off. The total amount of UV radiation is also reduced because the light is only on half the time.
“We’re now on the precipice of initiating our Phase III trial,” Dr. Rubinfeld concludes. “Based on the numbers from Phase II, we feel very good about the Phase III trial.”
The CXLens: Cross-linking on the Eye
While many researchers are primarily focused on improving the crosslinking procedure by allowing the epithelium to remain intact or finding ways to customize the result for keratoconus patients, one company, TECLens (Stamford, Connecticut), has developed an on-eye crosslinking system that uses scleral contact lenses, fiber optics and ultrasound to deliver the UV in a very controlled, targeted manner. The company believes this will eventually realize its long-held goal of pushing crosslinking beyond keratoconus into refractive correction for healthy eyes.
Roy S. Chuck, MD, PhD, chair of the Department of Ophthalmology and Visual Sciences at Albert Einstein College of Medicine in Bronx, N.Y., one of the cofounders of TECLens, explains the difference between a standard crosslinking treatment and one performed using TECLens’ CXLens device. “A standard crosslinking system uses an overhead lamp to irradiate the eye,” he says. “The UV lamp is on a large gantry-arm stand that takes up a lot of space in the clinic and needs a technician to continually steer the light to ensure proper targeting. The patient tries to lie perfectly still on the table for a half hour or so with a lid speculum in. This is uncomfortable and challenging for many patients, especially the younger ones.
“Instead of putting the UV lamp on a large stand, we’ve shrunk the control system to about the size of a laptop,” he continues. “We’ve moved the light emitter onto a disposable contact lens, the CXLens, that sits directly on the eye. This makes the procedure much more comfortable for the patient and more economical for the practitioner. You don’t need an eyelid speculum, and the patient can open and close their eye over the contact lens. The lens tracks with eye and head movement—no technician required. For refractive applications, our contact lens also carries a tiny ultrasound transducer to monitor the changing biomechanics of the cornea in real time, that will allow us to hit a specific target.”
Dr. Chuck explains that a contact lens reservoir is also used to apply the riboflavin to the eye. “You fill the vault space of the lens with riboflavin solution and place it on the eye for 20 to 30 minutes,” he says. “After riboflavin instillation, the reservoir lens is removed and the UV delivery lens is placed on the eye.” He adds that the CXLens process leaves the epithelium on. “It doesn’t seem to negatively impact the quality of the results, as long as you understand the interaction of the light and the riboflavin, and compensate for the drug that’s in the intact epithelium,” he says.
“During the TECLens procedure, patients can sit upright or lie down, whichever is most comfortable,” he points out. “TECLens’ scleral-lens patient interface allows both eyes to be treated simultaneously with different dosing specifications, saving time for both patient and practice. Additionally, because full time technician support isn’t necessary, a single operator can treat multiple patients at the same time, further increasing practice efficiency.
“In our pilot study in keratoconus, we used the 375-nm UVA light for 30 minutes to deliver a total dose of 7.2 J/cm2 to the central region of the cornea that contains the cone,” he explains. “For refractive correction, the treatment times will be likely be shorter, as the amount of crosslinking would be titrated to a patient’s specific needs.”
 |
| Photo: iVeena Delivery Systems. Click image to enlarge. |
Advantages of Miniaturization
Dr. Chuck notes that this approach has accomplished several things. “First, it makes the treatment less expensive,” he says. “Second, it’s more comfortable for the patient. Third, the scleral contact lens maintains the positioning of the light relative to the eye.
“In a traditional treatment, if you’re on the table underneath the lamp and you move out of position, the illumination is no longer aimed correctly,” he points out. “For keratoconus therapy, some small amount of patient movement can be tolerated, but for refractive correction, mis-targeting could cause unacceptable errors. The lamp-based systems try to compensate for patient movement by putting trackers in the system that turn off the light if the patient moves. But when you put the light on a scleral contact lens that sits on the eye, if the eye moves, the device moves with it. It becomes an ambulatory procedure. In fact, our clinical procedures were done in the exam room chair.”
Dr. Chuck explains that the first human study was recently done to ensure that the form, fit and function of the device is ready for healthy eyes. Ten patients with advanced keratoconus were treated. “It was a challenge to get some of the later patient visits done, given pandemic shut-downs,” he notes. “However, we were able to complete the pilot trial, and it confirmed that the system works well, is safe, and treats keratoconus effectively, as expected. The data were published last year in Translational Vision Science & Technology.10 Now we’re in the planning stages for our initial refractive studies and the larger keratoconus trials for approval.”
Dr. Chuck says the company has been working on several additional modifications that might enhance the CXLens system in the future, including a proprietary riboflavin formula intended to increase penetration through the cornea, and a highly oxygenated wetting fluid. In the meantime, it turns out that the results using standard elements such as commercially available riboflavin have been very good.
“Having sufficient oxygen is important to speed up a cross-linking treatment,” he says. “Hence, we came up with the idea of using a special highly oxygenated fluid as a wetting solution between the lens and the cornea. For the pilot trial, though, we used the commercially available solution and the results were good, so it’s possible we may not need to use the hyper-oxygenated fluid, or the proprietary riboflavin formula. Of course, we still plan to try them, to see if we can get even better results.”
For refractive indications, the on-eye UV delivery lens also incorporates a tiny ultrasound transducer that can provide real-time measurements of the changes in the cornea produced by the treatment. (For more on that, see Refractive Correction, below.)
Cross-linking Via an Eye Drop
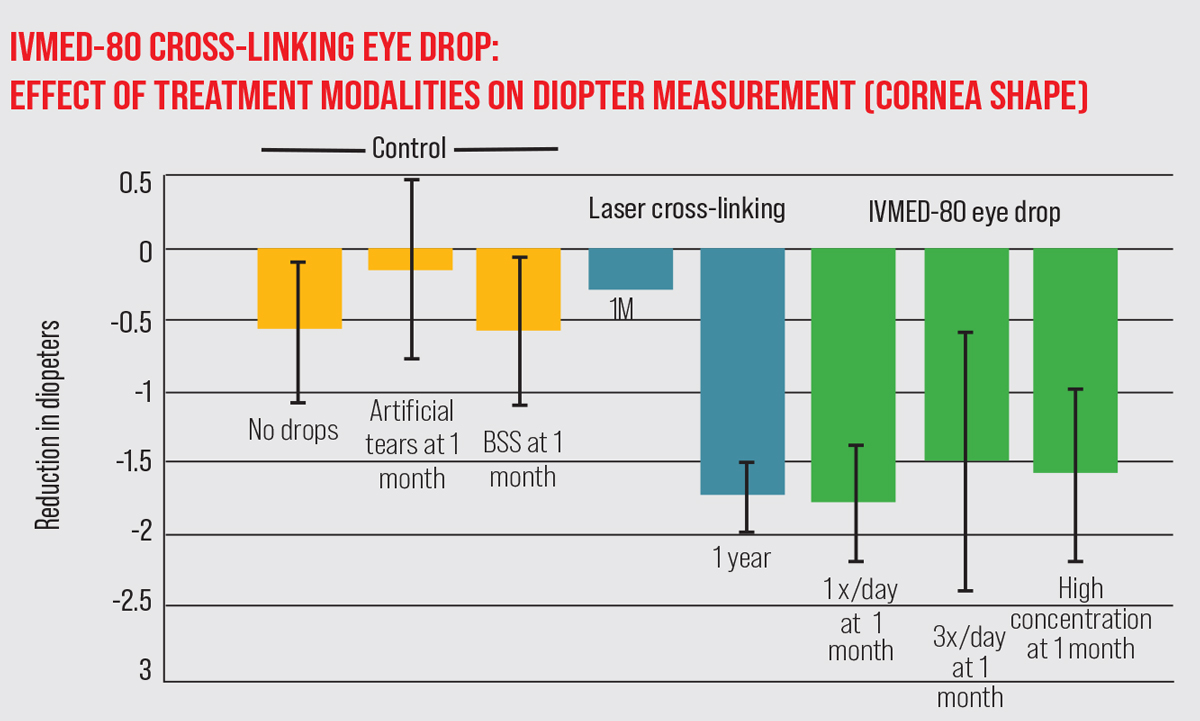
Another non-traditional approach to corneal cross-linking generates the cross-linking pharmacologically using an eye drop, rather than surgically. The developer, iVeena Delivery Systems in Salt Lake City, says that data from a Phase I/IIa study has demonstrated that the IVMED-80 drop strengthens the cornea and causes flattening. The drops were recently licensed by Glaukos for further clinical trials and development.
The drop was created by cornea specialist Bala Ambati, MD, president of the Pacific Clear Vision Institute in Eugene, Oregon, and a research professor at the University of Oregon. Dr. Ambati’s research revealed that lysyl oxidase, a natural enzyme in the cornea, mediates crosslinking. In fact, Dr. Ambati found a number of clinical studies that associated a deficiency of lysyl oxidase with keratoconus.11-17 Copper is a key factor in lysyl oxidase activity, so the IVMED-80 drop was designed to raise the amount of copper in the cornea; that, in turn, increases lysyl oxidase activity in corneal cells. Animal studies found no accumulation of copper in the blood, liver or kidneys after use of the drops.
The Phase I/IIa study of IVMED-80 involved 33 patients with keratoconus; one-third of the patients received placebo; one-third received IVMED-80 for about six weeks, and another third received the drop for 16 weeks.18 Results included:
- The patients who received the drug for 16 weeks ended up with a 1-D flatter Kmax; those in the placebo group saw a progression of 0.46 D of Kmax during the same period.
- On average, there was no regression in the 16-week group after stopping the drop.
- The drops cause no inflammation, stinging or redness.
“Cross-linking can be induced by surgery and ultraviolet light, or by the presence of an enzyme that’s normally present in most patients but is deficient in keratoconus patients,” notes Dr. Ambati. “Our drop increases the presence of that enzyme, and mass spectrometry on rabbit eyes has demonstrated that this results in increased cross-linking. Either surgery or drops can be used to induce cross-linking, but the drops have obvious advantages over traditional cross-linking because there’s no corneal scraping or pain for the patient.”
Dr. Ambati points out that using a drop makes sense, at least in part because surgical treatments don’t address the biochemical or genetic causes of the disease. “The cornea is living tissue that remodels,” he points out, noting that this makes regression a very real possibility. “Cross-linking just strengthens the tissue that’s present at the time of treatment. Because surgery isn’t curing the disease and comes with associated risks, trying a pharmacologic treatment first could make very good sense.”
Dr. Ambati believes that this approach wouldn’t eliminate the need for other forms of cross-linking treatment. “I think this drop would expand the pool of potential treatment candidates,” he says. “Keratoconus can range from mild to severe disease. If a patient isn’t ready for surgery or has concerns about surgery, this could be a great option.”
Dr. Ambati notes that if the drop is eventually approved, it’ll be up to the physician to decide what treatment to try first. “There could be patients for whom the drop doesn’t work, or patients for whom you want to do Intacs at the same time as cross-linking,” he points out. “There might be patients who are allergic to the drops or don’t want to take them. Like glaucoma, keratoconus is a chronic disease with both medical and surgical options. It’s up to the physician to figure out which option is best for which patient.”
In terms of what’s next, Dr. Ambati says Glaukos will be planning the next trial. “The Phase I/IIa has been done, testing safety and efficacy,” he says. “The FDA essentially gave us the green light to proceed with two randomized, controlled, Phase III studies. Now that Glaukos has licensed the drop, they can move the ball forward.”
Does evaluating cross-linking with kmax make sense? The current efficacy metric for cross-linking, commonly used to decide whether or not a treatment has worked, is the cornea’s Kmax value, the steepness at the steepest point on the cornea. “Michael Mrochen, PhD, once told me that the use of Kmax to evaluate the effectiveness of cross-linking may have begun with an early meeting he had with the FDA,” notes Roy S. Rubinfeld, MD, MA, medical director at Re:Vision in Rockville, Maryland, and Fairfax, Virginia, and a clinical professor of ophthalmology at Georgetown University Medical Center in Washington, DC. “The FDA asked how he had determined whether a cross-linking treatment was effective. He said his group was using the less-than-ideal method of measuring Kmax, which he strongly recommended not using. “The problem is that Kmax and quality of vision aren’t well correlated,” he continues. “It was my wife, an epidemiologist by training, who first pointed this out to me in 2010. I’d explained that we were using Kmax to determine whether a cross-linking procedure had succeeded. She looked over the numbers and pointed out that there was no correlation between vision improvement and Kmax. Since then, others have confirmed this. In one of our papers, Doyle Stulting, MD, demonstrated that patients who would be considered a failure because they had increased Kmax had mostly gained vision.2 Even Peter Hersh, MD, who did the studies for Glaukos/Avedro has noted that there’s no correlation. This is why our Phase II study of EpiSmart used corrected distance visual acuity as the primary study endpoint, with UCVA, Kmax and minimum corneal thickness as secondary endpoints.” Dr. Rubinfeld admits that the use of Kmax to gauge the effectiveness of cross-linking may take some time to fall out of common use. “Many patients have been sent back to me by the referring doctor,” he recalls, “with a note saying, ‘The patient’s Kmax is worse!’ I have to point out that the patient has gained three lines of vision. We have a disease that causes people to lose vision, so shouldn’t the metric for treatment success be whether the patient has lost or gained vision?” —CK |
Cross-linking to Treat Infection
Brent Kramer, MD, a cornea specialist at Vance Thompson Vision in Sioux Falls, South Dakota, has some experience using cross-linking to address corneal infections, including bacterial, fungal and Acanthamoeba keratitis. “Of course, I still defer to standard-of-care treatments such as drops, and sometimes when treating fungal and Acanthamoeba keratitis I use oral agents as well,” he says. “But for recalcitrant cases, it’s a tool I have in my toolkit if nothing else seems to be working.”
Dr. Kramer says that cross-linking for infection works two ways. “First, it attacks the bugs at the DNA and cell-wall level,” he says. “Second, you’re theoretically strengthening the cornea by forming covalent bonds.
“The data out there on this use of cross-linking is somewhat mixed,” he notes. “Patients I’d consider this for have fairly progressed disease and would likely need a corneal transplant in the future. But in those dire situations, the goal is to stabilize the cornea and avoid a therapeutic penetrating keratoplasty while the eye is hot and there’s an active infection. Treating the infection with cross-linking may decrease the risk of corneal perforation, although the jury is still out.
“That being said, the key to success in these cases is being aggressive about nailing down a diagnosis,” he continues. “Usually, if I can get a firm diagnosis with a culture or by other means, we’re able to treat with drops or oral medications effectively. I wouldn’t use cross-linking as an alternative to the standard therapies; I’d use it as an adjunct therapy when we need to slow the disease process down and we appear to be losing ground every day.
“Of course, it’s not for everyone,” he says. “First of all, patients who have any sort of herpes simplex aren’t good candidates for this, because we know that UV light can increase the activation of the herpes simplex virus. Even if the patient only has a history of HSV, I don’t think this would be a good treatment choice. Second, if the patient has a very deep infiltrate, I don’t think the UV light/riboflavin combination penetrates deep enough to get a completely effective treatment.
“Nevertheless, whether this will become a more mainstream approach probably depends on how the technology evolves,” he says. “It could become a very useful treatment when patients are unable to be compliant with drops, or are in parts of the world where drops aren’t available and can’t be compounded. Even here in the United States there are supply-chain disruptions. I think every cornea specialist has had a hard time getting their hands on natamycin for fungal keratitis cases in the past few years. In the future, cross-linking, or something similar could be a potential alternative treatment in these cases.
“The other issue is resistant bugs,” he adds. “I recently saw a patient with a pan-resistant Pseudomonas ulcer. By the time we’d seen the patient and obtained sensitivities there wasn’t much we could do. The patient eventually lost the eye. But if the resistance of the bug had been detected earlier, cross-linking might have made a difference.”
Refractive Correction
Because cross-linking reshapes the cornea to some extent, it was clear early on that there might be some potential to treat a small amount of refractive error using this technology—especially if the treatment could be customized for each patient. Glaukos/Avedro has produced a version of this treatment called PiXL, which incorporates their Mosaic device. PiXL allows customized delivery of different amounts of light and energy to different areas of the cornea. (PiXL is available in parts of Europe, but not approved in the United States.)
Many surgeons have expressed skepticism about this use of cross-linking, given that the refractive change created by cross-linking is inherently small and the resulting changes in corneal tissue are not yet predictable enough to make a precise refractive change possible. Some of the studies published in recent years shed some light on this idea’s potential:
- One study published in 2020 found that in healthy individuals age 25.6 ±3.6 years of age with low myopia, treating a 4-mm zone in a high-oxygen environment led to a significant improvement in UCVA (-0.45 ± 0.27 LogMAR) and MRSE (+0.99 ± 0.44 D) at one, six and 12 months.19 (At 12 months post treatment, endothelial cell count and BSCVA were unaltered.)
- Another study, published in 2021 compared two different customized PiXL treatment zones, a homogenous 4-mm zone and a 4-mm annular zone.20 Similar improvements in UDVA were seen for the homogeneous and annular protocols at one month: -0.52 vs. -0.49 logMAR (p=0.91). Both groups also showed a similar mean improvement of 1 D in MRSE (p=0.17). Reduction in mean keratometry was -0.8 D and 0 D respectively (p<0.001). The treatment effect remained stable throughout 24 months. No adverse events were reported, but at one week, participants reported less ocular discomfort with the annular protocol.
- A third randomized, single-masked study involving 27 patients (54 eyes), was reported in 2022.21 This study compared two different PiXL protocols. One eye was treated in a central annular zone of 4 mm
(UV light 30 mW/cm2, with the light on for one second, off for one second); the fellow eye was treated in a 3.5-mm annular zone (45 mW/cm2, half a second on, one second off). Results were assessed through a 24-month follow-up. Findings included:
— The 3.5-mm protocol produced a larger improvement in UDVA: 0.52 vs. 0.38 logMAR, p=0.003, and in MRSE: +1.25 D vs. 1 D, p=0.037.
— A transient reduction in LCVA was larger with the 3.5-mm protocol (p<0.01).
— No reductions in ECC or BSCVA were noted.
— The 3.5-mm protocol rendered less subjective ocular discomfort post-treatment.
— No adverse events were noted.
One surgeon who has some experience performing the PiXL treatment is Anders Behndig, MD, a professor in the Department of Clinical Sciences and Ophthalmology at the Umeå University Hospital in Umeå, Sweden. (Dr. Behndig participated in the three trials mentioned above.)
Dr. Behndig notes that so far, his group has only used the PiXL treatment protocol in randomized clinical trials, not in clinical routine. “For a select group of patients with low grade myopia—between -0.75 D and -2 D—the results can be comparable to those of other possible treatments,” he says. “Right now, the effect of PiXL is limited; we had difficulty getting more than 2 D of effect. The precision of the treatment may be another issue, although that’s not a major problem if you select the right patients.”
Miltos Balidis, MD, PhD, a current Mosaic/PIXL user at the Institute of Ophthalmology & Microsurgery Ophthalmica Eye Clinic in Thessaloniki, Greece, has participated in trials relating to treating myopia and presbyopia. “With the tested treatment protocol, we’ve successfully treated low myopia up to 1.25 D,” he says. “Unfortunately, the response to treatment was variable. It didn’t work in every case. And so far, the results using current presbyopic treatment profiles have been disappointing.”
Refractive Correction with the CXLens
The CXLens on-eye cross-linking system (described earlier) also plans to eventually perform cross-linking-based refractive change. To make that possible, the scleral lens UV delivery system also incorporates a tiny ultrasound transducer that can provide real-time measurements of the changes in the cornea produced by the treatment.
“One of the challenges of this type of treatment is the difficult-to-predict corneal response,” Dr. Chuck, cofounder of TecLens, the company developing the CXLens, points out. “The problem is that every eye is a little different; you can put the same illumination on two different eyes and get two different effects. The way to eliminate this variability is to directly measure the biomechanical changes that are happening in the cornea during the treatment. That’s what our ultrasound monitor can do: track the changes in the cornea, in real time, as the illumination proceeds.
“There have been other attempts to do this,” he continues. “One that seems to have fallen out of favor involves using an optical monitor to measure Brillouin scattering. You shine a light on the cornea and monitor tiny changes in the light’s wavelength to determine how the cornea is changing. The major drawback of that approach is that it can’t be done in real time. Using ultrasound, each pulse takes microseconds, and with the device positioned inside the contact lens, you can monitor the corneal changes in real time. This data allows the clinical treatment to be coupled to a computational pre-plan customized for each patient’s topography and underlying corneal biomechanics. That’s been the great hope for crosslinking since the early days; precise control of the reshaping, instead of just doing the procedure and hoping for the best. We believe our system has all the tools to make this happen for a huge number of patients seeking refractive correction.”
Dr. Chuck says that so far the company has successfully used the embedded ultrasound monitor in the lab, using human donor corneas. “It hasn’t been validated in the clinic yet,” he notes. “We hope to do that soon. We look forward to making CXL refractive correction a real option for patients, without having to remove tissue, cause pain, cause post-LASIK ectasia, subject patients to LASIK smoke, and so forth,” he says.
Dr. Rubinfeld has equity interest in CXL Ophthalmics. Dr. Chuck is co-founder of TECLens, creator of the CXLens. Dr. Ambati is the president and co-founder of iVeena. Dr. Balidis is a consultant for Glaukos/Avedro. Drs. Behndig and Kramer report no financial ties to anything discussed in this article. (Dr. Rubinfeld can be reached at Doctor@revisedeye.com.)
1. Epstein RJ, Belin MW, Gravemann D, Littner R, Rubinfeld RS. EpiSmart crosslinking for keratoconus: A Phase 2 study. Cornea, Sept 29 2022. Online ahead of print.
2. Stulting RD, Trattler WB, Woolfson JM, et al. Corneal crosslinking without epithelial removal. J Cataract Refract Surg 2018:44:1363–1370.
3. Vaidya NS, Daneshmand A, Epstein RJ, et al. Pachymetric assessment after EpiSmart epithelium-on cross-linking for keratoconus and post-surgical ectasia. Ophthalmol 2022;16:1829-1835.
4. Hersh PS, Stulting RD, Muller D, Durrie DS, Rajpal RK; United States Crosslinking Study Group. United States multicenter clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology 2017;124:9:1259-1270.
5. Gore DM, O’Brart D, French P, Dunsby C, Allan BD. Transepithelial riboflavin absorption in an ex vivo rabbit corneal model. Invest Ophthalmol Vis Sci 2015;56:8:5006-11.
6. Rubinfeld, RS, Glenwood, GG, Talamo, JH, Parsons, EC. The effect of sodium iodide on stromal loading, distribution and degradation of riboflavin in a rabbit model of transepithelial corneal crosslinking. Clinical Ophthalmology 2021;15:1985–94.
8. Rubinfeld R, Stulting RD, Gum G, Talamo J. Quantitative analysis of corneal stromal riboflavin concentration without epithelial removal. J Cataract Refract Surg 2018;44:2:237-242.
9. Rubinfeld RS, Caruso C, Ostacolo C. Corneal cross-linking: The science beyond the myths and misconceptions. Cornea 2019;38:6:780-790.
10. Dackowski EK, Logrono JB, Rivera C, et al. Transepithelial corneal crosslinking using a novel ultraviolet light-emitting contact lens device: A pilot study. Transl Vis Sci Technol 2021;10:5:5.
11. Bykhovskaya Y, Li X, Epifantseva I, et al. Variation in the lysyl oxidase (LOX) gene is associated with keratoconus in family-based and case-control studies. Invest Ophthalmol Vis Sci 2012;53:7:4152-7.
12. Dudakova L, Jirsova K. The impairment of lysyl oxidase in keratoconus and in keratoconus-associated disorders. J Neural Transm (Vienna) 2013;120:6:977-82.
13. Hasanian-Langroudi F, Saravani R, et al. Association of lysyl oxidase (LOX) polymorphisms with the risk of keratoconus in an Iranian population. Ophthalmic Genet 2015;36:4:309-14.
14. Dudakova L, Liskova P, et al. Changes in lysyl oxidase (LOX) distribution and its decreased activity in keratoconus corneas. Exp Eye Res 2012;104:74-81.
15. Dudakova L, Palos M, et al. Validation of rs2956540:G>C and rs3735520:G>A association with keratoconus in a population of European descent. Eur J Hum Genet 2015;23:11:1581-3.
16. Zhang J, Zhang L, et al. Association of common variants in LOX with keratoconus: A meta-analysis. PLoS One 2015;10:12:e0145815.
17. Shetty R, Kumar NR, Khamar P, et al. Bilaterally asymmetric corneal ectasia following SMILE with asymmetrically reduced stromal molecular markers. J Refract Surg 2019;35:1:6-14.
18. Molokhia S, Muddana SK, Hauritz H, et al. IVMED 80 eye drops for treatment of keratoconus in patients—Phase 1/2a. Invest Ophth Vis Sci 2020;61,2587.
19. Fredriksson A, Näslund S, Behndig A. A prospective evaluation of photorefractive intrastromal cross-linking for the treatment of low-grade myopia. Acta Ophthalmol 2020;98:2:201-206.
20. Näslund S, Fredriksson A, Alm A, Rehnman JB, Behndig A. Treatment effect with 2 photorefractive intrastromal cross-linking protocols in low-grade myopia through 24-month follow-up. Acta Ophthalmol 2021;99:5:519-526.
21. Näslund S, Rehnman JB, Fredriksson A, Behndig A. Comparison of two annular photorefractive intrastromal cross-linking protocols in high oxygen for low-grade myopia through 24-month follow-up. Acta Ophthalmol 2022;100:5:549-558.