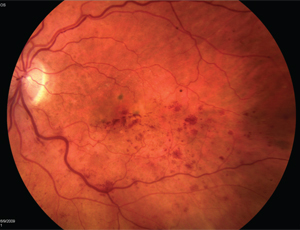
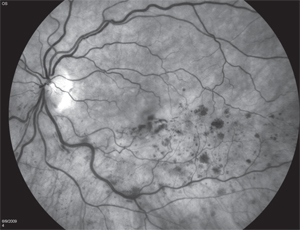
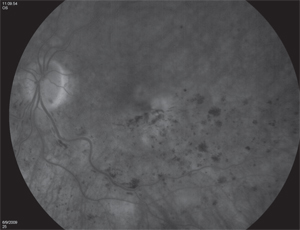
Chicago-based surgeon Seenu Hariprasad, MD, points out that only two pharmacologics are Food and Drug Administration-approved for use to treat all retinal vein occlusions: Ozurdex (dexamethasone intravitreal implant) and Lucentis. Eylea (aflibercept intravitreal injection) is also FDA-approved, but only for the treatment of central retinal vein occlusion.
Dr. Capone says deciding on a drug is done empirically: “You try one class of drug to see if it works. If it doesn’t, you try the other. If it only works partially, you combine them. There is considerable individual variability with regard to response. The knee-jerk reflex nowadays is to go with an anti-VEGF agent first. If that doesn’t work at all, switch to a steroid. If it works partially, the general practice is to switch to another anti-VEGF agent, seeking a better response in a given individual. After that, we’ll try steroids either alone or in combination.”
Both Lucentis and Avastin have been shown to effectively treat retinal vein occlusion and have similar visual and anatomic outcomes. A recent retrospective study included 81 patients with retinal vein occlusion and macular edema who were naïve to anti-VEGF therapy.1 Twenty-six eyes were treated with ranibizumab (Lucentis), 33 eyes were treated with bevacizumab (Avastin), and 22 eyes were treated with bevacizumab and then switched to ranibizumab. The main outcome measure was change in visual acuity at three months, six months and at the final visit.
|
Dexamethasone intravitreal implants have also been shown to be a safe and effective treatment option. A recent study evaluated the safety and efficacy of one or two treatments over 12 months in eyes with macular edema related to branch or central retinal vein occlusion.2
This study included 1,256 patients with vision loss caused by macular edema associated with retinal vein occlusion. At baseline, 421 patients received a dexamethasone 0.7-mg implant, 412 received a dexamethasone 0.35-mg implant, and 423 received a sham implant. At day 180, patients could receive a dexamethasone 0.7-mg implant if their best-corrected visual acuity was less than 84 letters or if their retinal thickness was greater than 250 μm, and 997 patients received this implant. Except for cataract, the incidence of ocular adverse events was similar in patients who received their first or second dexamethasone implant. Over 12 months, cataract progression occurred in 90 of 302 phakic eyes (29.8 percent) that received two dexamethasone implant 0.7-mg injections compared with five of 88 sham-treated phakic eyes (5.7 percent). Cataract surgery was performed in four of the 302 (1.3 percent) phakic eyes that received two implants and one of 88 (1.1 percent) eyes that received the sham implant.
An improvement in best-corrected visual acuity of 15 letters or more from baseline was achieved by 30 percent of patients 60 days after the first dexamethasone implant and by 32 percent of patients 60 days after the second dexamethasone implant.
Dr. Hariprasad believes combining pharmaceuticals is very advantageous. “There are some patients in whom I go straight to a combination approach, and there are other patients who are suboptimal responders in whom we will try combination therapy,” Dr. Hariprasad says.
A recent study found that bevacizumab combined with dexamethasone implants produced greater improvements in macular thickness than bevacizumab therapy alone and required fewer bevacizumab injections in cases of both branch and central retinal vein occlusion.3
The study included 30 eyes that were randomly assigned to receive either combination therapy or monotherapy with bevacizumab. All patients received intravitreal bevacizumab at baseline, followed one week later by dexamethasone implants or sham injections. Monthly bevacizumab injections were given if the central subfield thickness was less than 250 µm, and the combined group received a second implant after four or five months if the central subfield thickness was less than 250 µm.
At six months, patients receiving combined therapy required fewer bevacizumab re-injections compared to those receiving monotherapy (two versus three). The combined therapy group also experienced greater mean reductions in central subfield thickness (-56 µm versus +45 µm) and were more likely to have resolved all edema, which was considered a central subfield thickness less than 250 µm (seven of 11 eyes versus two of 14 eyes). Mean visual acuity changes from baseline were similar between groups.
|
He believes that a better approach is to use an anti-VEGF agent and promptly gauge by the presence and magnitude of a response whether the patient should be treated exclusively with an anti-VEGF or whether a steroid should be brought in. “A decision can be made earlier in the game than is conventional practice as to whether an adjunctive or alternative therapeutic agent would be appropriate for a patient with vein occlusion that is only partially responsive or largely unresponsive,” Dr. Capone says.
Los Angeles-based surgeon David Boyer, MD, agrees. “If patients don’t have the response that I would like, I add the steroids very early,” he says. “Other ophthalmologists like to wait until after four, five or six injections. If I’m not seeing the response I want after a couple of injections of anti-VEGF and I add a steroid, it doesn’t mean I’m not going to use anti-VEGF after that, but I can add a steroid for a better response.”
Michael Singer, MD, from San Antonio, Texas, typically initiates treatment with Lucentis in cases of branch retinal vein occlusion because he conducted a study that found that ranibizumab appears to have a greater effect than bevacizumab in the short-term of decreasing macular edema.4
This retrospective study included 64 patients with retinal vein occlusions. Half received injections of bevacizumab, and half received injections of ranibizumab. Central macular thickness and best-corrected visual acuity were obtained at baseline, at two weeks (just prior to the dexamethasone intravitreal implant), and at six weeks. At the two-week examination, the bevacizumab group had a mean central macular thickness reduction of 26.2 ±3.4 percent compared with a 47 ±3.5 percent reduction with ranibizumab. At six weeks, there was a 31.6 ±3.2 percent central macular thickness reduction with bevacizumab versus 52 percent ±3.2 percent with ranibizumab. At two weeks, 15 (9 percent) bevacizumab patients and 25 (78.1 percent) ranibizumab patients achieved a central macular thickness of less than 300 μm, and at six weeks, 18 (56.3 percent) bevacizumab patients compared to 30 (93.8 percent) ranibizumab patients achieved a central macular thickness less than 300 μm. Visual acuity was not significantly different between the two groups at any time interval.
“I like the results of this study because vein occlusion patients are a lot younger than macular degeneration patients as a general rule, and they need better vision sooner,” Dr. Singer says. “With central retinal vein occlusion, it gets a little more complicated. If it’s more ischemic, I’m much more likely to use Eylea, because the GALILEO and COPERNICUS studies included central retinal vein occlusions in their study design, so aflibercept’s effectiveness has been shown.5,6 The CRUISE study7 excluded these patients based on the inclusion criteria for the study, so ranibizumab’s effectiveness has not been demonstrated in a clinical trial setting,” Dr. Singer adds.
According to Dr. Boyer, triamcinolone has been shown to be helpful in central retinal vein occlusions, and Ozurdex has been shown to be helpful in both central retinal vein and branch retinal vein occlusion. “We usually start with an anti-VEGF drug, and if I get a great response, meaning that it dries out and vision improves, then I probably would continue the anti-VEGF, hoping that I could eventually get to a treatment and extend protocol and eventually not need to treat the patient,” he says.
Surgical Treatments
According to Dr. Capone, there has been a rise and fall of interest in the surgical management of venous occlusion, whether it is branch retinal vein occlusion with arterial venous sheathotomy, or central retinal vein occlusion with radial optic neurotomy. However, these approaches have not withstood the test of time.
Dr. Hariprasad believes that focal laser treatment has a role in the treatment of branch retinal vein occlusion, but its use in central retinal vein occlusion is more controversial. “If focal laser treatment is not working, we always have vitrectomy surgery with internal limiting membrane peeling in our back pocket as a last resort,” he explains.
A recent study has shown that the combination of Ozurdex and macular grid laser is synergistic in increasing best-corrected visual acuity and lengthening the time between injections in patients with branch retinal vein occlusion.5
Another treatment option for branch retinal vein occlusion is short-duration PASCAL macular photocoagulation, which appears to be safe and provide anatomical improvement.6
“Right now, surgery is rarely used and is reserved for people who, with chronic treatment over a long period of time, don’t respond,” Dr. Boyer says. “If we can duplicate the surgery, meaning that a study could be done that shows the benefit of surgery over anti-VEGF therapy, then I think more people would utilize it, but there are risks involved with surgery and no standardization of the treatment. If surgery could be standardized and more reliable with a low complication rate, I definitely think it would be something that we would have to consider.”
Systemic Treatments
Interestingly, systemic treatment may play a role in resolving macular edema. Three recent cases of macular edema associated with retinal vein occlusion improved after successful treatment of systemic hypertension alone.7
The first case was a 72-year-old woman with a central retinal vein occlusion who had macular edema in her left eye and visual acuity of 20/50. Her blood pressure was 169/96 mmHg, and she was prescribed a calcium blocker. One month after the initiation of treatment, her blood pressure was decreased, macular edema was reduced and her visual acuity improved to 20/20.
The second case was a 62-year-old woman with branch retinal vein occlusion. Her visual acuity was 20/40, and her blood pressure was 165/97 mmHg. After six weeks of taking medication to treat her systemic hypertension, the macular edema associated with retinal vein occlusion had decreased and her VA improved to 20/20.
The third case was a 71-year-old man with branch retinal vein occlusion. His visual acuity was 20/50, and his blood pressure was 165/87 mmHg. One month after initiation of treatment for systemic hypertension, his macular edema had disappeared and his visual acuity improved to 20/20.
All of these cases had non-ischemic retinal vein occlusion by fluorescein angiography, and none developed ischemic changes for at least one year.
The authors of this study recommend that blood pressure be measured in all patients with macular edema before initiating treatment with an intravitreal anti-VEGF agent.
Future
According to Dr. Hariprasad, the future of treating retinal vein occlusion looks bright. “There are other sustained-delivery steroid devices that are being investigated, and sustained delivery of steroids and anti-VEGF agents will be a godsend for these patients. We are also looking at wide-field angiography to see if peripheral nonperfusion has a role in the management of this disease,” he explains. REVIEW
1. Yuan A, Ahmad BU, Xu D, et al. Comparison of intravitreal ranibizumab and bevacizumab for the treatment of macular edema secondary to retinal vein occlusion. Int J Ophthalmol 2014;7(1):86-91.
2. Haller JA, Bandello F, Belfort R Jr, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion: twelve-month study results. Ophthalmology 2011;118:2453-2460.
3. Maturi RK, Chen V, Raghinaru D, Bleau L, Stewart MW. A 6-month, subject-masked, randomized controlled study to assess efficacy of dexamethasone as an adjunct to bevacizumab compared with bevacizumab alone in the treatment of patients with macular edema due to central or branch retinal vein occlusion. Clin Ophthalmol 2014;8:1057-1064.
4. Singer MA, Cohen SR, Groth SL, Porbandarwalla S. Comparing bevacizumab and ranibizumab for initial reduction of central macular thickness in patients with retinal vein occlusions. Clin Ophthalmol 2013;7:1377-1383.
5. Korobelnik JF, Holz FG, Roider J, et al; GALILEO Study Group. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: One-year results of the Phase 3 GALILEO Study. Ophthalmology 2014;121:202-208.
6. Heier JS, Clark WL, Boyer DS, et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: Two-year results from the COPERNICUS Study. Ophthalmology 2014;121:1414-1420.
7. Brown DM, Campochiaro PA, Singh RP, et al; CRUISE Investigators. Ranibizumab for macular edema following central retinal vein occlusion: Six-month primary end point results of a phase III study. Ophthalmology 2010;117:1124-1133.
8. Pichi F, Specchia C, Vitale L, et al. Combination therapy with dexamethasone intravitreal implant and macular grid laser in patients with branch retinal vein occlusion. Am J Ophthalmol 2014;157:607-615.
9. Pitcher JD 3rd, Liu T, Prasad PS, Schwartz SD, Hubschman JP. Short-duration focal pattern grid photocoagulation for macular edema secondary to branch retinal vein occlusion. Semin Ophthalmol 2012;27(3-4):69-72.
10. Kida T, Morishita S, Kakurai K, Suzuki H, Oku H, Ikeda T. Treatment of systemic hypertension is important for improvement of macular edema associated with retinal vein occlusion. Clin Ophthalmol 2014;8:955-958.