A new clinical study from the DRCR Retina Network, supported by the NEI, found that early initiation of anti-VEGF therapy reduced the development of more advanced diabetic retinopathy at two years. Also at two years, however, the injections’ effect on vision was similar to standard therapy used to treat the onset of late disease.
The interim findings of the Protocol W clinical trial were published in JAMA Ophthalmology in late March. Raj K. Maturi, MD, of Indiana University and the protocol chair for the study, says the study will help to determine whether there’s any benefit to treating patients who haven’t yet reached proliferative disease stages.
“The DRCR.net Protocol S demonstrated that anti-VEGF injections were noninferior to panretinal photocoagulation for treatment of PDR,” he explains. “In our study, we looked at the moderate and severe NPDR categories to see if early treatment with intravitreal anti-VEGF injections would prevent any of the complications of DR that happen with more advanced disease, and if it’d prevent vision loss. Our data clearly show that the chances of developing DME or some of the severe forms of DR are reduced by almost a factor of three with the early use of anti-VEGF agents.”
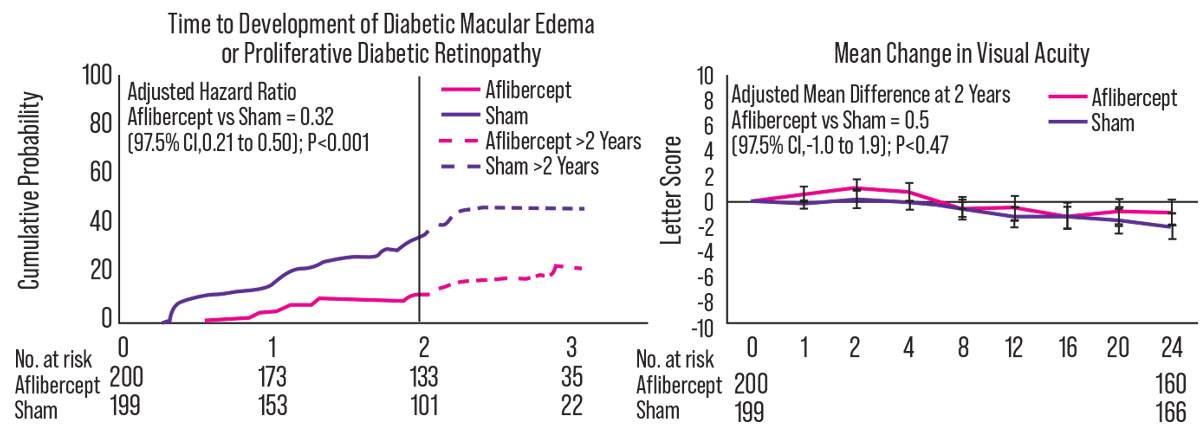
The four-year study includes 328 patients (399 eyes), randomized to receive either 2 mg aflibercept (n=200) or a placebo (n=199). At two years, 16.3 percent of the treated group and 43.5 percent of the placebo group developed center-involved DME with vision loss or PDR (p<0.001). Additionally, aflibercept eyes demonstrated significant differences in DR severity (defined as a change of two or more steps from baseline) compared to the sham group: 44.8 percent vs. 13.7 percent of eyes improved (p<0.001) and 5.2 percent vs. 12.4 percent eyes worsened (p=0.03), respectively. Regardless of treatment group, patients who progressed were treated with aflibercept, according to standard clinical care.
 |
|
Left panel: Time from randomization to development of PDR or center-involved DME (composite outcome). The vertical line represents the end of the two-year visit window (815 days after randomization) when two-year cumulative probabilities were estimated. Hazard ratio includes all available data through four years and was adjusted for DR severity at the screening visit, study eye laterality and correlation between eyes of participants with two study eyes. Figure was truncated at the timepoint at which data from less than 20 eyes in each treatment group were available. Right panel: Mean change in visual acuity over two years. Treatment group difference was calculated after multiple imputation of missing data and covariate adjustment for DR severity and baseline VA at the screening visit, study eye laterality, and correlation between eyes of participants with two study eyes. |
The Protocol W study also examined functional vison as a coprimary endpoint. “Our data very clearly showed that there was no difference in vision between the groups,” Dr. Maturi says. Baseline visual acuity was 20/25 or better, and the two-year adjusted mean difference in VA change was 0.5 letters (-0.9 ±5.8 letters for aflibercept vs. -2 ±6.1 letters for sham [p=0.47]).
Medicare provides complete coverage for any patient with DR to get preventive treatment, but there’s a potential cost. “The early treatment group required about eight injections over two years, while the treatment-deferred group received an average of one treatment over the entire two-year period,” Dr. Maturi explains. “The non-treatment group got far fewer injections and didn’t lose any vision, even though the DR looks like it would have progressed, in many cases.
“At four years, we’ll see whether the benefit of early injections outweighs the risks,” he continues. “Right now, the DRCR recommends watching these patients every four months until they develop PDR-like changes or DME. That’s when we suggest treatment be initiated. Of course, there will always be individual cases where earlier treatment may be indicated.”
Though the FDA has already approved aflibercept for DR at q8-week dosing regardless of retinopathy level, Regeneron is seeking FDA approval to market the drug with a q16-week dosing interval, which may make it more competitive with other anti-VEGF agents. However, Dr. Maturi notes that currently, “using aflibercept at less-frequent intervals isn’t an issue, since it’s approved for use up to q8 weeks.”
Disclosures: Dr. Maturi receives financial support for clinical trials from Allergan, Genentech-Roche, Allegro Pharmaceuticals, Boehringer-Ingelheim and Aerpio Therapeutics, LLC.
In Brief New Johnson & Johnson Vision Phaco Machine Approved Johnson & Johnson Vision announced FDA 510 clearance and the CE mark for its next-generation phacoemulsification device, the Veritas Vision System. The company says the system “features technologies that enable surgeons to guide through any lens density with less surge and more stability.” It also provides advancements in ergonomics to enhance usability during cataract surgery. The machine will be available for purchase later this year.
Topline Results From Phase III NOV03 Trial for Dry-eye Drug Bausch + Lomb announced topline data from the first Phase III trial (GOBI trial) evaluating the investigational topical drug NOV03 (perfluorohexyloctane) aimed at treating the signs and symptoms of dry-eye disease associated with meibomian gland dysfunction. In the topline data, the drug met both of its co-primary endpoints.
New Viscoelastic Approved Bausch + Lomb announced the FDA approved ClearVisc. ClearVisc contains sorbitol, a chemical agent that the company says has been shown in a laboratory study to provide protection from free radicals. The company adds that free radicals can contribute to corneal damage. In a multicenter, randomized, clinical study of 372 subjects, ClearVisc met its primary safety and efficacy endpoints and was demonstrated to be non-inferior to Viscoat, B+L says. |