Toxoplasmosis is one of the most frequently identifiable causes of uveitis worldwide. In fact, Toxoplasma gondii infection is the most common cause of infectious posterior uveitis in non-immunocompromised individuals, and second only to cytomegalovirus retinitis in patients with HIV/AIDS.1-3
Toxoplasma gondii is an obligatory intracellular parasite. While systemic signs and symptoms of infection are less common in healthy adults, such findings may be present in newborns and immunocompromised patients. Sexual reproduction of the parasite occurs in small intestinal epithelial cells of the cat, with subsequent fecal elimination of oocysts. Once ingested by other animals, which serve as intermediate hosts, the oocysts rupture to release tachyzoites, which ultimately travel to target tissues to become tissue cysts or bradyzoites.4-6
Clinical Manifestation, Diagnosis
Traditionally, it was thought that most active ocular toxoplasmosis represented reactivation of congenital toxoplasmosis acquired transplacentally from the mother. Recently, however, it has been shown that acquired infections occur more frequently than previously suspected.7
Toxoplasma infection is asymptomatic in most immunocompetent patients and, when it occurs, is usually benign and self-limited. The infection may be much more severe, however, in the fetus and immunocompromised patients.3
The clinical presentation of ocular toxoplasmosis depends on patient age, and the location, size and severity of retinochoroiditis. Ocular manifestations include floaters and blurred vision. Decreased visual acuity may occur as a result of macular involvement or severe vitreous inflammation. In immunocompromised patients, the clinical presentation may be rather atypical.8
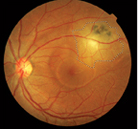
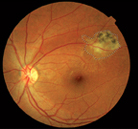
Toxoplasmic retinochoroiditis is a recurrent disease in two-thirds of patients.9-11 An active toxoplasmic retinochoroiditis is whitish and moderately exudative with ill-defined borders and involves the macula in a majority of patients.12 Mild to moderate anterior segment inflammation may or may not be a presenting feature;13 vitreous inflammation is virtually always present. Retinal vasculitis in the vicinity of an active lesion or in the distant retina may also be seen.8
Retinochoroiditis in patients with HIV/AIDS may show atypical features, such as large confluent areas of retinochoroidal necrosis and/or active bilateral lesions.8
Optic neuritis, punctate outer re-tinitis, neuroretinitis, papillitis and pseudoretinitis are other atypical manifestations of this disorder. During the healing process, retinochoroidal shunt and even choroidal neovascularization, gliosis and vitreous tractional bands may develop.8,14
|
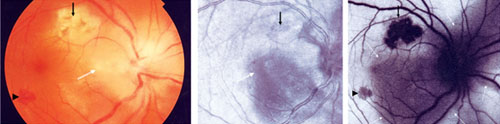
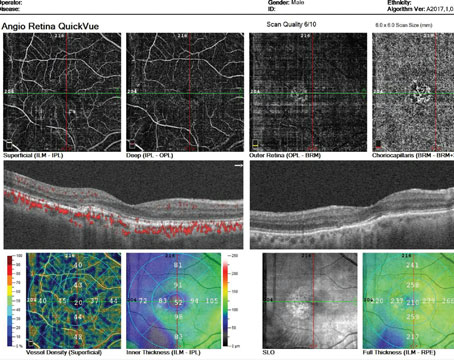
Fluorescein angiography and indo-cyanine green angiography findings in toxoplasmic retinochoroiditis are nonspecific.8 Two recent non-invasive photography techniques, i.e., infrared and autofluorescence, may further enhance our ability to determine the extent of retinochoroiditis. Autofluorescence imaging of toxoplasmic retinochoroidal scars demonstrates a dark area (hypo-autofluorescence) due to lack of functional retinal pigment epithelium (See Figure 1). Acute, active lesions may also show hypo-autofluorescence due to the presence of overlying retinal edema. In fact, autofluorescence imaging can be used to monitor the effect of medical therapy, since it better demonstrates resolution of active retinal edema.16
Differential Diagnosis
Recurrent toxoplasmic retinochoroiditis adjacent to a scar area may be confused with serpiginous choroiditis. Necrotizing retinitis due to CMV, herpes simplex virus, herpes zoster virus, fungal retinitis (candidiasis, blastomycosis), septic retinitis, ocular toxocariasis, sarcoidosis, syphilis and tuberculosis are other diagnoses to exclude when considering toxoplasmosis. Punctate outer retinal toxoplasmosis is an atypical form of ocular toxoplasmosis that may be confused with other white dot syndromes.8
Prevention
Transmission can occur by ingesting oocysts, tachyzoites, tissue cysts or bradyzoites. In addition to contaminated food, water source contamination is increasingly recognized as a source of infection. The disease can also be acquired by transfusion of whole blood or leukocytes, by organ transplant, or accidentally in laboratory workers.17-20
Prevention of the initial infection is the most effective way of reducing Toxoplasma-related morbidity. Raw meat, raw eggs, unwashed vegetables and unpasteurized milk should not be consumed.
Preventive strategies include cooking and freezing meat, washing fruits and vegetables,21 hand washing8 and avoiding the use of contaminated water.22 Blood transfusions and organ transplants from sero-positive donors should be avoided.8 Prevention of disease transmission is also crucial for immunocompromised individuals and sero-negative pregnant women.4,8 Cat vaccination may interrupt the parasite life cycle.6,21
|
Treatment
The goal of treatment is to arrest the multiplication of the parasite during the active period of retinochoroiditis and to minimize damage to the retina and optic nerve. Despite being a self-limiting disease in most instances, Toxoplasma infection may cause decreased vision secondary to optic nerve or macular involvement, and severe vitreous inflammation.23 Patients with the following characteristics are considered by most to be appropriate treatment candidates:
- optic nerve involvement—either direct or within two disc diameters;
- a lesion within the temporal arcades or threatening the arcade vessels;
- a large lesion with subretinal hemorrhage and/or serous retinal de-tachment regardless of location;
- severe vitreous inflammation;
- loss of more than two lines in visual acuity;
- persistent retinochoroidal inflam-mation for more than one month;
- toxoplasmic retinochoroiditis in the first year of life;
- a newborn diagnosed with con-genital toxoplasmosis regardless of the presence or absence of ocular lesions; and
- any lesion in an immunocom-promised host.8
Since active lesions, even far from the macula, may be associated with loss of visual acuity due to macular edema, intense vitritis, macular tractions or detachment, treating any active lesions may be indicated, particularly given the emergence of safer treatment regimens. Furthermore, tachyzoites released from reactivated tissue cysts may spread to other sites in the retina. For this reason, some believe that treatment of any active lesion may be associated with a decreased overall tachyzoite burden, hence diminishing the risk of recurrences.8 That said, an evidence-based systematic review of the literature disclosed a lack of evidence to support routine antibiotic treatment for acute toxoplasmic retinochoroiditis.24 Moreover, it should be emphasized that none of the treatment regimens discussed below have been compared in a randomized, controlled fashion over the natural course of the disease.
|
The most common treatment for ocular toxoplasmosis is so-called “classic therapy,” which consists of pyrimethamine and sulfadiazine plus corticosteroids. Classic therapy consists of an initial dose of 75 to 100 mg of pyrimethamine daily for two days followed by a 25- to 50-mg dose daily and a 2- to 4-g of sulfadiazine daily for two days, followed by a 500-mg to 1-g dose every six hours as well as 5 mg of folinic acid daily for four to six weeks. Oral prednisolone (1 mg/kg daily) is given from the third day of therapy and tapered over two to six weeks.10,15,21 Alternative treatment regimens include quadruple drug therapy (classic regimen plus clindamycin), as well as single or combined use of clindamycin, trimethoprim/sulfamethoxazole, spiramycine, minocycline, azithromycin, atovaquone and clarithromycin.24-32Laser photocoagulation and vitrectomy has also been used in some settings.8,14
Classic treatment is not without risk, however. Pyrimethamine administration requires weekly monitoring of blood cell and platelet counts, as well as the co-administration of folinic acid to protect against leukopenia and thrombocytopenia. Similarly, sulfadiazine may cause a severe allergic reaction, which can be life-threatening in some patients. Moreover, the cost of these drugs is high and they are not readily available in some areas. In addition, use of these drugs in pregnant women is not safe, and there is no liquid formula of such drugs for pediatric patients. Compliance can also be difficult, considering that patients need to receive up to 10 pills per day.1,15,25,32,33 Given these issues, safer and simpler therapies for ocular toxoplasmosis have received increasing attention.
Treatment of toxoplasmosis retinochoroiditis with intravitreal injection of clindamycin and dexamethasone has had promising effects.32,34-42 Intravitreal drug administration, by bypassing ocular barriers, can deliver a high concentration of drug directly to the intraocular tissues, while avoiding systemic exposure and its attendant risk of complications. Having a good intracellular penetration, clindamycin provides a high intracellular concentration against T. gondii, which is an intracellular parasite. It can cause an intracellular/extracellular ratio of 43 compared to some other antibiotics such as erythromycin or levofloxacin, which have ratios of 14 and 6, respectively.32,43,44 Intravitreal injection of 1.5 mg clindamycin was found to be non-toxic to the retina, with a half-life of 5.6 days. Following 1-mg intravitreal injection of clindamycin, its concentration remained ≥1.6 µg/mL for about 40 hours, which is higher than the 50-percent inhibitory concentration for T. gondii.32,41,42
|
In a recent randomized clinical trial, it has been shown that intravitreal injection of clindamycin and dexamethasone is an acceptable alternative to the classic treatment in ocular toxoplasmosis. The intravitreal approach provides the patient more convenience, a better safety profile, greater drug availability and fewer follow-up visits and hematologic evaluations. In this study, the mean number of injections was 1.6 with a range of one to three injections performed biweekly. Weekly injections of the same drugs have also been suggested.45 Newly acquired toxoplasmosis may be better treated with systemic therapy, a point supported by the fact that lesion size reduction has been noted to be greater following classic vs. intravitreal therapy in IgM-positive patients.32
Systemic corticosteroids can reduce damage to ocular tissues. About 82 percent of the members in an American Uveitis Society survey utilized systemic corticosteroids in the management of ocular toxoplasmosis.45 Given the widespread use of intravitreal dexamethasone in the management of endophthalmitis,44 intravitreal dexamethasone has also gained acceptance as an adjunct treatment for ocular toxoplasmosis.32
Newborns and infants with con-genital ocular toxoplasmosis should receive treatment within the first year of life with a combination of pyrimethamine, sulfadiazine and folinic acid.8
In immunocompromised patients, the treatment regimen is partially modified. It should be emphasized that any active retinal lesion in immunocompromised patients demands treatment because the risk of dissemination of the infection and related complications is high. Pyrimethamine has an antagonistic activity against ziduvudine, an antiretroviral agent used in the treatment of AIDS. For this reason, and because of the risk of drug-induced bone marrow suppression, pyrimethamine sometimes may be avoided or used in lower dosage in the treatment regimen of patients with HIV/AIDS who receive highly active antiretroviral therapy (HAART). Lifetime maintenance therapy including either a lower dosage of pyrimethamine combined with sulfadiazine or clindamycin or trimethoprim/sulfamethoxazole is crucial to prevent relapse and dissemination of the infection.8
The recurrence rate of toxoplasmic retinochoroiditis correlates inversely with the period of follow-up. Other host factors and the particular strain of T. gondii may also play a role.1,8,32 In two separate studies at our institution, the recurrence rates within two years were determined to be between 5.9 percent and 10.3 percent.28,32 It has been shown in one study that long-term intermittent treatment with trimethoprim (160 mg)/sulfamethox-azole (800 mg) every three days may reduce the recurrence rate.46
In summary, ocular toxoplasmosis is the most common cause of infectious posterior uveitis in many countries. Although there is no effective therapy to eradicate the organism, treatment is accompanied by resolution of active infection in the vast majority of cases. Recent studies have demonstrated that this goal may be achieved with safer regimens, such as oral trimethoprim/sulfamethoxazole or intravitreal clindamycin/dexamethasone. REVIEW
Dr. Soheilian is a professor of ophthalmology in the Ophthalmology De- partment and Ophthalmic Research Center, Labbafinejad Medical Center, Shaheed Beheshti Medical University, and practices at Negah Eye Hospital, both in Tehran. Dr. Ramezani is an associate professor of ophthalmology at the Labbafinejad Center and practices at Imam Hossein Medical Center, also in Tehran. Roham Soheilian is a medical student at Shiraz University of Medical Sciences, Kish, Iran.
Contact Dr. Soheilian at Ophthalmic Research Center, Labbafinejad Medical Center, Pasdaran Ave. Boostan 9 St. Tehran 16666, Iran; fax: +98 21 22562138; or e-mail:
masoud_soheilian@yahoo.com. The authors have no proprietary or commercial interest in any material discussed in this article.
1. Soheilian M, Heidari K, Yazdani S, et al. Patterns of uveitis in a tertiary eye care center in Iran. Ocul Immunol Inflamm 2004;12(4):297-310.
2. Perkins ES. Ocular toxoplasmosis. Br J Ophthalmol 1973;57(1):1-17.
3. Burnett AJ, Shortt SG, Isaac-Renton J, et al. Multiple cases of acquired toxoplasmosis retinitis presenting in an outbreak. Ophthalmology 1998;105:1032-7.
4. Holland GN, O’Conner GR. Ocular toxoplasmosis. In: Tasman W, ed. Duane’s foundations of clinical ophthalmology. Philadelphia: Lippincott Williams & Wilkins, 2004.
5. Smith LA. Diagnostic parasitology. In: Mahon CR, Manuselis G, eds. Textbook of diagnostic microbiology, 2nd ed. Philadelphia: Saunders, 2000.
6. Yanoff M, Fine BS. Ocular Pathology, 5th ed. St. Louis: Mosby, 2002.
7. Gilbert RE, Stanford MR. Is ocular toxoplasmosis caused by prenatal or postnatal infection? Br J Ophthalmol 2000;84(2):224-6.
8. Da Mata AP, Orifice F. Toxoplasmosis. In: Foster CF, Vitale AT, eds. Diagnosis and treatment of uveitis. Philadelphia: Saunders, 2002.
9. Araujo F, Slifer T, Kim S. Chronic infection with Toxoplasma gondii does not prevent acute disease or colonization of the brain with tissue cysts following reinfection with different strains of the parasite. J Parasitol 1997;83(3):521-2.
10. Rothova A, Meenken C, Buitenhuis HJ, et al. Therapy for ocular toxoplasmosis. Am J Ophthalmol 1993;115:517-23.
11. Ghosh M, Levy PM, Leopold IH. Therapy of Toxoplasmosis Uveitis. Am J Ophthalmol 1965;59:55-61.
12. Mets MB, Holfels E, Boyer KM, et al. Eye manifestations of congenital toxoplasmosis. Am J Ophthalmol 1996;122:309-24.
13. La Hey E, Rothova A, Baarsma GS, et al. Fuchs’ heterochromic iridocyclitis is not associated with ocular toxoplasmosis. Arch Ophthalmol 1992;110:806-11.
14. Nussenbleatt RB, Whitcup SM. Uveitis. Fundamentals and Clinical Practice, 3rd ed. St. Louis: Mosby, 2004.
15. Dodds E. Ocular toxoplasmosis: clinical presentation, diagnosis and therapy. In: Focal points: Clinical Modules for ophthalmologists. San Francisco American Academy of Ophthalmology, 1999; v. XVII.
16. Soheilian M, Rezaei Kanavi M, Sadoughi MM, Azimzadeh A. Ocular Toxoplasmosis In: Ashok G, Prost Marek E, Rajvardhan A, Crouch Eric R, eds. Surgical & Medical Management of Pediatric Ophthalmology. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd, 2007.
17. Silveira C, Belfort R, Jr., Burnier M, Jr., Nussenblatt R. Acquired toxoplasmic infection as the cause of toxoplasmic retinochoroiditis in families. Am J Ophthalmol 1988;106:362-4.
18. Engstrom RE, Jr., Holland GN, Nussenblatt RB, Jabs DA. Current practices in the management of ocular toxoplasmosis. Am J Ophthalmol 1991;111:601-10.
19. Rothova A. Ocular involvement in toxoplasmosis. Br J Ophthalmol 1993;77(6):371-7.
20. Couvreur J, Thulliez P. Acquired toxoplasmosis of ocular or neurologic site: 49 cases. Presse Med 1996;25(9):438-42.
21. Holland GN, O’Connor R, Belfort R, Remington JS. Toxoplasmosis. St. Louis: Mosby Year Book, 1996.
22. Isaac-Renton J, Bowie WR, King A, et al. Detection of Toxoplasma gondii oocysts in drinking water. Appl Environ Microbiol 1998;64(6):2278-80.
23. Bosch-Driessen LE, Berendschot TT, Ongkosuwito JV, Rothova A. Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmology 2002;109:869-78.
24. Stanford MR, See SE, Jones LV, Gilbert RE. Antibiotics for toxoplasmic retinochoroiditis: an evidence-based systematic review. Ophthalmology 2003;110:926-31; quiz 31-2.
25. Tabbara K. Toxoplasmosis. In: Tasman W, Jaeger E, eds. Duane’s clinical ophthalmology. Philadelphia: JB Lippincott, 1995; v. 4.
26. Martin WG, Brown GC, Parrish RK, et al. Ocular toxoplasmosis and visual field defects. Am J Ophthalmol 1980;90:25-9.
27. Hovakimyan A, Cunningham ET Jr. Ocular toxoplasmosis. Ophthalmol Clin North Am 2002;15(3):327-32.
28. Soheilian M, Sadoughi MM, Ghajarnia M, et al. Prospective randomized trial of trimethoprim/sulfamethoxazole versus pyrimeth-amine and sulfadiazine in the treatment of ocular toxoplasmosis. Ophthalmology 2005;112:1876-82.
29. Rothova A, Bosch-Driessen LE, van Loon NH, Treffers WF. Azithromycin for ocular toxoplasmosis. Br J Ophthalmol 1998;82(11):1306-8.
30. Lopez JS, de Smet MD, Masur H, et al. Orally administered 566C80 for treatment of ocular toxoplasmosis in a patient with the acquired immunodeficiency syndrome. Am J Ophthalmol 1992;113:331-3.
31. Holland GN, O’Conner GR, Belfort R Jr, Remington JS. Toxoplasmosis. In: Pepose JS, Holland GN, Wilhelmuis KR, eds. Ocular infection and Immunity. St. Louis: Mosby, 1996;1183-223.
32. Soheilian M, Ramezani A, Azimzadeh A, et al. Randomized Trial of Intravitreal Clindamycin and Dexamethasone versus Pyrimethamine, Sulfadiazine, and Prednisolone in Treatment of Ocular Toxoplasmosis. Ophthalmology. 2011 Jan;118(1):134-41. Epub 2010 Aug 12.
33. Holland GN. Reconsidering the pathogenesis of ocular toxo-plasmosis. Am J Ophthalmol 1999;128:502-5.
34. Kishore K, Conway MD, Peyman GA. Intravitreal clindamycin and dexamethasone for toxoplasmic retinochoroiditis. Ophthalmic Surg Lasers 2001;32:183-92.
35. Martinez CE, Zhang D, Conway MD, Peyman GA. Successful management of ocular toxoplasmosis during pregnancy using combined intraocular clindamycin and dexamethasone with systemic sulfadiazine. Int Ophthalmol 1998;22(2):85-8.
36. Sobrin L, Kump LI, Foster CS. Intravitreal clindamycin for toxoplasmic retinochoroiditis. Retina 2007;27:952-7.
37. Nozik RA. Results of treatment of ocular toxoplasmosis with injectable corticosteroids. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol 1977;83(5):811-8.
38. O’Connor GR. Manifestations and management of ocular toxoplasmosis. Bull N Y Acad Med 1974;50(2):192-210.
39. Elkins BS, Holland GN, Opremcak EM, et al. Ocular toxoplasmosis misdiagnosed as cytomegalovirus retinopathy in immuno-compromised patients. Ophthalmology 1994;101:499-507.
40. Ferguson JG, Jr. Clindamycin therapy for toxoplasmosis. Ann Ophthalmol 1981;13:95-100.
41. Paquejt JT, Peyman GA. Intravitreal clindamycin phosphate in the treatment of vitreous infection. Ophthalmic surgery 1974;5(3):34-9.
42. Peyman GA, Charles HC, Liu KR, et al. Intravitreal liposome-encapsulated drugs: A preliminary human report. Int Ophthalmol 1988;12(3):175-82.
43. Mandell GL, Coleman E. Uptake, transport, and delivery of antimicrobial agents by human polymorphonuclear neutrophils. Antimicrob Agents Chemother 2001;45(6):1794-8.
44. Peyman GA, Lee PJ, Seal D. Endophthalmitis Diagnosis and Management. London: Taylor & Francis, 2004; 81-100.
45. Lasave AF, Diaz-Llopis M, Muccioli C, et al. Intravitreal clindamycin and dexamethasone for zone 1 toxoplasmic retinochoroiditis at twenty-four months. Ophthalmology 2010;117:1831-8.
46. Silveira C, Belfort R, Jr., Muccioli C, et al. The effect of long-term intermittent trimethoprim/sulfamethoxazole treatment on recurrences of toxoplasmic retinochoroiditis. Am J Ophthalmol 2002;134:41-6.