Stem cells, currently under investigation for the treatment of age-related macular degeneration and other retinal disorders, are characterized by the ability to differentiate into multiple cell lineages, and an unlimited self-renewal capacity. These traits make them excellent candidates as potential treatments for various diseases. To date, however, no stem cell-based therapy for retinal disease has been approved by the U.S. Food and Drug Administration, though there are several c
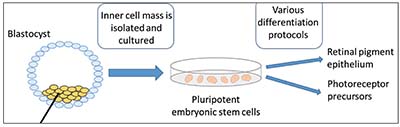 |
| Figure 1. Embryonic stem cell-based therapy. The inner cell mass is isolated from the blastocyst and cultured. The pluripotent embryonic stem cells are then differentiated into retinal pigment epithelium, photoreceptor precursors or other cell types using various methods. |
andidates in development. In this article, we’ll focus on human studies of stem cell-based ocular therapy.
Stem Cell Primer
Pluripotent stem cells (PSCs), by definition, are able to differentiate into all endodermal, mesodermal and ectodermal lineages. Human embryonic stem cells (hESCs) were first cultured in 1998 and have the potential to differentiate into all cell types (Figure 1). They are a promising source for stem cell-based therapy but, like fetal progenitor cells, raise potential ethical considerations. Induced pluripotent stem cells (iPSCs) are a subtype of pluripotent stem cells that originate from a differentiated cell source, such as skin fibroblasts or blood cells (Figure 2); they may be considered less controversial, and may negate some immunological issues associated with hESC-based therapies. Somatic stem cells, such as bone marrow, adipose, central nervous system and umbilical stem cells, are different than ESC- or iPSC-based therapies because they’re not pluripotent, but can generate some of the cell types of their host organ. While they normally assume a regenerative role in their host organ (i.e., corneal limbus epithelial stem cells), somatic stem cells typically assume a trophic role in stem cell therapy (Figure 3).
It turns out that the eye is a good candidate for stem cell clinical research, given the unmet therapeutic need, the relatively immune-privileged site and the clear ocular media that facilitates direct visualization of transplanted cells. Furthermore, the size of the eye requires smaller quantities of therapeutic tissue in comparison to other organs.
In the eye, stem cells can potentially serve two different therapeutic roles: regenerative or trophic. For example, stem cells have the potential to replace or regenerate tissue, such as retinal ganglion cells in glaucoma, or retinal pigment epithelium in retinitis pigmentosa or AMD-related geographic atrophy (GA). They can alternatively or simultaneously assume a trophic role, producing growth factors and cytokines, such as brain-derived neurotrophic factor, that have a supportive paracrine effect on local structures within the macula. (It’s worth noting that most current approaches using somatic stem cells to treat retinal disease use an intravitreal delivery method, in contrast to subretinal transplantation.1)
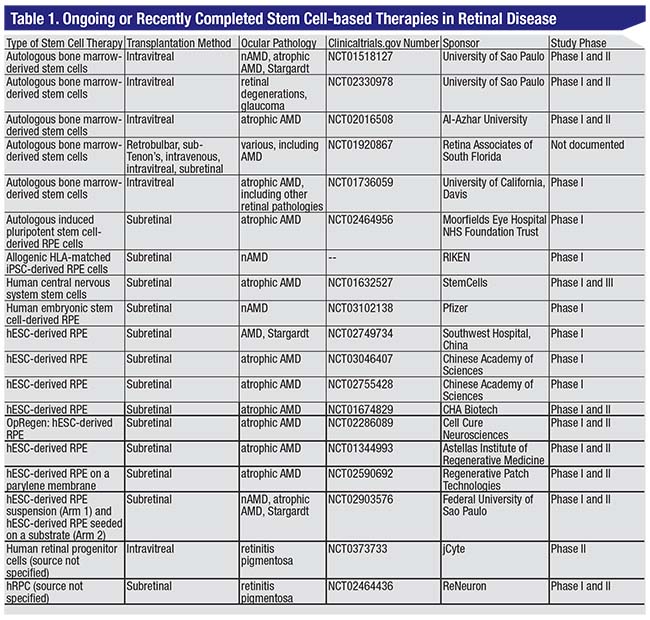 |
RPE Transplantation
Transplant of retinal pigment epithelium cells is a popular application of stem cell therapy in ophthalmology, with researchers taking different approaches:
• Embryonic stem cells in RPE transplantation. The first human studies of stem cell-based RPE transplants in AMD and Stargardt Disease were published in 2012.1 Steven D. Schwartz, MD, of the Stein Eye Institute in Los Angeles, and his colleagues performed two prospective clinical trials of subretinal transplantation of hESC-derived RPE cells in nine patients with Stargardt macular dystrophy and nine with atrophic AMD.2 Following surgery combined with immunosuppression, 72 percent of patients had increased subretinal pigmentation at the location of the transplant, suggesting the presence of the injected cells.2 No serious adverse outcomes were observed in visual acuity, visual field, static perimetry, electroretinography or reading speed, and there were no signs of acute rejection. Even after four years, none of the eyes developed abnormal growth suggestive of a teratoma, a tumor composed of two or more germ layers which could originate from stem cells, and no eyes developed proliferative vitreoretinopathy or a retinal detachment.2,3
In 2015, Won Kyung Song, MD, of Korea’s Bundang Medical center, and co-workers published preliminary results of subretinal hESC-derived RPE transplantation in two patients with advanced atrophic AMD and two patients with Stargardt disease.4 Si
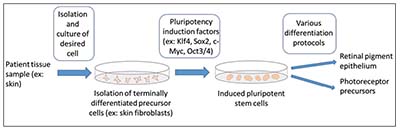 |
| Figure 2. The process of inducing pluripotency followed by differentiation of cells into the desired cell type. First, a tissue is harvested from the adult patient. The tissue is then processed and the desired cell type isolated and cultured. The cells are then induced into pluripotency through the introduction of particular factors and growth conditions. Once pluripotency is established, the cells can be differentiated into the desired cell type, such as retinal pigment epithelium or photoreceptor precursor cells. |
milar to Dr. Schwartz’s study, no patients developed teratomas, graft rejection, PVR or a significant visual decline. However, this study did note some challenges expected with surgery surrounding retinotomy sites, as well as intolerance of immunosuppression in one patient.4
A recent Phase I trial of hESC transplants on a coated, synthetic basement membrane in two patients with advanced exudative AMD was suggestive of survival of the graft through 12 months. The study highlighted the feasibility of transplantation of RPE cells on the synthetic membrane, but also identified perioperative challenges, including a
retinal detachment due to PVR, dislocation of a fluocinolone implant used for immunosuppression and worsening of diabetes from the use of oral steroids.5
• Induced pluripotent stem cells in RPE transplantation. The use of iPSC-derived RPE transplants in human trials has lagged behind the use of ESCs. The first human trial using iPSC-derived RPE subretinal transplants was initiated by RIKEN, a research institute in Kobe, Japan, in September 2014.6 A 70-year-old Japanese woman became the first person to receive an iPSC-derived therapy for any indication.7 She didn’t receive immunosuppression, in contrast to ESC-derived RPE transplantation studies.2,4 The subject demonstrated no adverse ocular effects at one year, the transplanted sheets remained intact and her vision decline had stabilized.8 This study was suspended after mutations were observed in a second subject’s iPSCs, which weren’t detectable in the patient’s original fibroblasts.6
In 2016, RIKEN planned to resume the study, with a significant modification: Instead of autologous cells, its researchers are investigating human leukocyte antigen-matched allogenic iPSC-derived RPE cells.6
The use of autologous cells is costly and may require up to three months to develop from harvesting to intraocular implantation.7 In contrast, the use of allogenic cells facilitates verification of genomic stability and expedites the time from patient selection to implantation.6
A potential downside of using allogenic cells, however, is the increased risk of rejection due to the presentation of non-self antigens and the possible need for immunosuppression.
Trophic Roles for Stem Cells
Human intravitreal, autologous bone marrow-derived mononuclear transplantation was first published in 2008, and demonstrated no significant safety issues in an eye with advanced diabetic retinopathy with optic nerve atrophy and retinal detachment.9 The same group expanded the study to include two additional patients, including a patient with advanced atrophic AMD. Two of the three patients underwent pars plana vitrectomy followed by intravitreal transplantation of suspended cells, and the third patient had the transplant injected into silicone oil. In all patients, the cells disappeared within four weeks, and other than a mild increase in intraocular pressure (absolute readings of 15 to 30 mmHg), no adverse events were published.10
A separate group investigated the intravitreal injection of autologous CD34+ bone marrow stem cells in various retinal pathologies. In contrast to the studies mentioned above, this group didn’t perform PPV prior to intravitreal injection. The transplant was tolerated well with no intraocular inflammation or tumor formation. At six mon
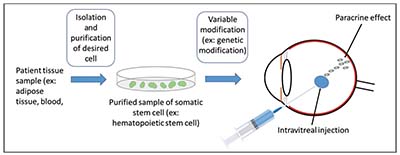 |
| Figure 3. Somatic stem cell therapy. First, tissue is harvested from the patient and the desired cell is isolated and purified. The cells can then variably be modified and are typically injected intravitreally where they have a paracrine effect. Alternatively, the tissue can be injected subretinally and some investigators are investigating periocular injections. |
ths postoperatively, five of the six study eyes demonstrated VA stabilization, but one eye developed progression of AMD-related GA with a visual decline.11 Overall, these studies suggest the basic tolerability of the procedure, with further studies needed to clarify safety and efficacy. Other human studies have investigated the use of autologous bone marrow-derived mononuclear cells in patients with retinitis pigmentosa, retinal vein occlusion and cone-rod dystrophy.12-14
Postoperatively, patients injected with mesenchymal or hematopoietic stem cell-based therapies may be at increased risk of proliferation of cells within the vitreous. For example, following intravitreal injection of CD34-positive stem cells, a 71-year-old female patient developed a visually significant epiretinal membrane within four months.15 Another patient, a 60-year-old man with Stargardt disease, developed a retinal detachment two months following subretinal injection of autologous mesenchymal stem cells.16 Most recently, Ajay E. Kuriyan, MD, at the University of Rochester Medical Center in Rochester, New York, published an account of a tragic case series of three patients who experienced vitreous proliferation, retinal detachment and profound loss of vision following adipose tissue-derived mesenchymal stem cell intravitreal transplants at one center in Florida.17 One of the patients saw the treatment on www.clinicaltrials.gov, and erroneously interpreted the listing as a clinical trial with government approval and oversight, even though the center billed patients directly for the therapy (which is very unusual in a true clinical trial). It’s imperative for physicians to appropriately educate patients about the possible downsides of unproven stem cell therapies being conducted outside of a true controlled clinical trial setting.
In conclusion, stem cell-based therapies have intriguing potential, but this field is still in its infancy. In the last several years, ESC-, iPSC-, and somatic stem cell-based therapies have advanced from in vitro and animal models to human trials with limited efficacy data. The major limitation of applying stem cell-based therapies to patients with AMD and similar pathologies is the chronic and complex disease process. For example, years of oxidative stress, an impaired inflammatory state with complement activation, and aging with choriocapillaris atrophy and ischemia create a microenvironment in AMD that challenges successful tissue replacement, engraftment and survival. Furthermore, given the polarity of RPE cells, transplanting sheets of cells with a scaffold, instead of suspensions, may be more physiologic, and some groups are consequently developing this concept further. Furthermore, surgical technique and immunosuppression will require additional clarification. REVIEW
Peter Bracha, MD, is chief resident and Thomas A. Ciulla, MD, MBA, is a volunteer clinical professor of ophthalmology at Indiana University School of Medicine. Dr. Ciulla also serves on the board of directors of Midwest Eye Institute and has an employment relationship with Spark Therapeutics. Neither Dr. Bracha nor Dr. Ciulla have financial interests in the subject matter.
1. Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 2012;379:9817:713-20.
2. Schwartz S, Regillo CD, Lamet BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 2015;385:9967:509-16.
3. Schwartz, SD, Tan G, Hosseini H, Nagiel A. Subretinal Transplantation of Embryonic Stem Cell-Derived Retinal Pigment Epithelium for the Treatment of Macular Degeneration: An Assessment at 4 Years. Invest Ophthalmol Vis Sci 2016;57:5:1-9.
4. Song, WK, Park KM, Kim HJ, et al. Treatment of macular degeneration using embryonic stem cell-derived retinal pigment epithelium: Preliminary results in Asian patients. Stem Cell Reports 2015;4:5:860-72.
5. da Cruz L, Fynes K, Georgiadis O, et al. Phase I clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol 2018. 36:4:328-337.
6. Garber K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat Biotechnol 2015;33:9:890-1.
7. Chakradhar S. An eye to the future: Researchers debate best path for stem cell-derived therapies. Nat Med 2016;22:2:116-9.
8. Mandai M. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N Engl J Med 2017;376:11:1038.
9. Jonas JB. Intravitreal autologous bone marrow-derived mononuclear cell transplantation: a feasibility report. Acta Ophthalmol 2008;86:2:225-6.
10. Jonas JB. Intravitreal autologous bone-marrow-derived mononuclear cell transplantation. Acta Ophthalmol 2010;88:4:e131-2.
11. Park SS, Bauer G, Abedi M, et al. Intravitreal autologous bone marrow CD34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings. Invest Ophthalmol Vis Sci 2014;56:1:81-9.
12. Siqueira RC, Messias A, Voltarelli JC, et al. Intravitreal injection of autologous bone marrow-derived mononuclear cells for hereditary retinal dystrophy: a phase I trial. Retina 2011;31:6:1207-14.
13. Siqueira RC, Messias A, Voltarelli JC, et al. Resolution of macular oedema associated with retinitis pigmentosa after intravitreal use of autologous BM-derived hematopoietic stem cell transplantation. Bone Marrow Transplant 2013;48:4:612-3.
14. Siqueira RC, Rubens C, Siqueira AM, Gurgel VP, et al. Improvement of ischaemic macular oedema after intravitreal injection of autologous bone marrow-derived haematopoietic stem cells. Acta Ophthalmol 2015;93:2:e174-6.
15. Kim JY, You YS, Kim SH, et al. Epiretinal membrane formation after intravitreal autologous stem cell implantation in a retinitis pigmentosa patient. Retin Cases Brief Rep 2017;11:3:227-231.
16. Leung EH, HW Flynn, TA Albini, et al. Retinal detachment after subretinal stem cell transplantation. Ophthalmic Surg Lasers Imaging Retina 2016;47:6:600-1.
17. Kuriyan AE, TA Albini, JH Townsend, et al. Vision loss after intravitreal injection of autologous “stem cells” for AMD. N Engl J Med 2017;376:11:1047-1053.



