Retinopathy of prematurity has been a recognized disease entity and a leading cause of childhood blindness since the 1950s. By 1984, the first internationally agreed upon classification system was developed. The international classification of retinopathy of prematurity (ICROP) became essential in standardizing ROP disease findings for advancements in clinical research, physician communication and patient care worldwide.1 As technologies for neonatal care advanced, the clinical manifestations of ROP changed. ICROP evolved to include disease classifications like aggressive posterior ROP (AP-ROP) and pre-plus disease.2 Now, physicians have realized that the definition needs to be tweaked a bit further, based on the impacts of intravitreal anti-VEGF medications to the disease course and ROP disease presentation in the developing world.3 Here, I’ll review the latest guidelines to help you effectively evaluate infants with ROP.
The New Guidelines
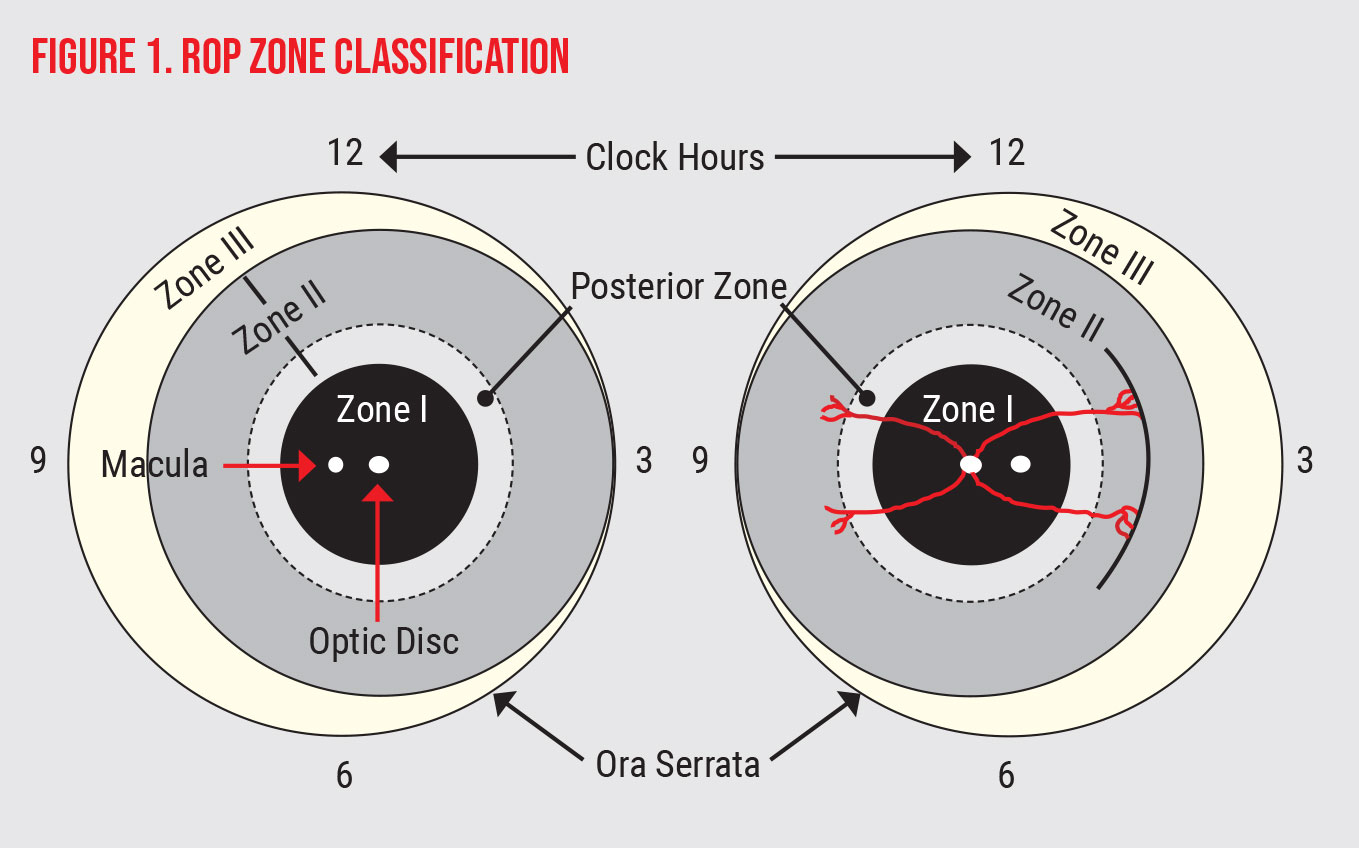
In the latest revision of ICROP, published in the fall of 2021 by the National Eye Institute’s Michael F. Chiang, MD, and colleagues, there are additional nuanced changes. This international panel of experts advised the following updated recommendations when classifying the zone of disease (For ease of reference, the zones appear in Figure 1, below).
First, there’s now a special classification of disease, termed a “notch,” for ROP that’s more anterior in most sectors and more posterior in only one to two clock hours (usually at the horizontal meridian). The physicians advise to denote the zone of the eye as the more posterior zone but with the qualifier “secondary to notch.”
 |
In the guidelines, “plus disease” is defined as “the appearance of dilation and tortuosity of retinal vessels,” and “preplus disease” is defined by “abnormal vascular dilation and/or tortuosity insufficient for plus disease.”
Specifically, in terms of classification of “plus” disease the panel advises the following updates:
- vessels in the area of zone I (an area twice the distance of the optic nerve to the fovea—See Fig. 1) should be used to define the level of plus disease, rather than peripheral vessel appearance;
- continue to view plus disease as a spectrum; and
- iris vessel engorgement, poor pupil dilation, peripheral retinal vessel engorgement and vitreous haze all indicate advanced disease but aren’t required for the diagnosis of plus disease.
ROP Stage
In terms of ROP disease stage classification, the ROP experts have several recommendations.
Aggressive posterior ROP (AP-ROP), will now be renamed Aggressive-ROP, or A-ROP. According to the new guidelines, this change was done to better encompass the disease spectrum with an emphasis on severity and speed of progression. A-ROP doesn’t need to be present in very posterior zones for diagnosis, as is the case in larger infants, but should still be viewed and managed as aggressive disease, similar to the way we managed AP-ROP.
Signs of A-ROP include rapid development of stage 3 with plus disease, extremely anomalous vasculature with shunting and vessel loops present, and flat-appearing stage 3 without line or ridge demarcation.
Stage 5 retinal detachment classifications have been revised to be more amenable to standard eye exams. Stage 5A denotes a total retinal detachment with the optic nerve visible, an open funnel configuration to the detachment. Stage 5B denotes a total retinal detachment with the optic nerve NOT visible, a closed funnel. Stage 5C includes the findings of Stage 5B along with anterior segment anomalies like a shallow anterior chamber, iridolenticular adhesions and corneal opacity.
Disease Descriptors
More detailed disease descriptors, including disease regression, reactivation and long-term sequelae, were suggested by the ICROP ROP expert panel as well.
Disease regression may be complete or incomplete, spontaneous or after treatment. It may also be followed by clinically stable incomplete vascularization of the retina, termed “persistent avascular retina.” PAR should be described in terms of zone and extent, if present.
ROP reactivation after treatment is becoming increasingly prevalent with the greater use of anti-VEGF therapies. When it occurs, the committee advises classifying reactivated disease with the traditional zone and stage identifiers, but with “reactivation” as an additional descriptor.
With decades of preterm infants surviving well into late adulthood (usually defined as 65+ years old), clinicians are continuing to recognize additional complications. The latest ICROP guidelines emphasize the need to recognize long-term sequelae of ROP, such as late retinal detachment, retinoschisis, PAR, macular anomalies, retinal vessel anomalies and secondary angle-closure glaucoma.
Screening Guidelines
In addition to accurate disease classification from ICROP, standardized screening guidelines for ROP have also advanced care and improved visual outcomes for premature infants. The American Association of Pediatrics, in collaboration with the American Association for Pediatric Ophthalmology and Strabismus, and the American Academy of Ophthalmology, periodically update and publish the national standardized guidelines for screening and treatment of ROP in the United States that have been in place since the late 1990s.4 The latest guidelines from 2018 advise:
- Screening all infants less than or equal to 1,500 g birthweight or 30 weeks or less gestational age at birth.
- Additional screening for infants 1,500 to 2,000 g birthweight or greater than 30 weeks gestational age may be performed based on infant co-morbidities or neonatologist concern.
- First eye exam is advised to be performed at 31 weeks adjusted gestational age or four weeks after birth, whichever is later. However, earlier exams for infants 22 to 24 weeks gestational age at birth or those with higher comorbidities could be considered for an exam at the practitioner’s discretion.
- Subsequent exams are performed every few days to every two to three weeks, depending on the zone and stage of disease.
- Acute screening can be terminated when the infant reaches any of the following criteria: full vascularization; zone III ROP without prior zone I or II disease; or adjusted gestational age of 45 weeks or more with no current stage 3 disease in zone II or any disease in zone I.
- For infants treated with anti-VEGF agents, it’s advised to maintain acute screening until an adjusted gestational age of 65 weeks.
On the Horizon
ROP stages Stage 1 — Mild disease, formation of demarcation line. Stage 2 — Moderate disease, elevation and increased width of a demarcation line to form a ridge. Stage 3 — Severely diseased, neovascularization typically extraretinal and emanating from a ridge the exception being in zone I where stage 3 may be intraretinal. Stage 4 — Partially detached retina. |
It’s been repeatedly recognized that the current workforce of ROP examiners can’t keep up with the demand for ROP screening exams.5 In an effort to potentially reduce the burden of exams, authors of several reports on neonatal algorithms are challenging the traditional ROP screening guidelines to better fine tune which infants need screening exams.
In 2019, researchers applied previously reported G-ROP screening criteria to a new cohort of infants across the United States and Canada.6 The stricter criteria included infants with one or more of the following: gestational age less than 28 weeks at birth or birth weight less than 1,051 g; weight gain less than 120 g between ages 10 to 19 days; weight gain less than 180 g between ages 20 to 29 days; weight gain less than 170 g between ages 30 to 39 days; or hydrocephalus.
In this study, G-ROP screening criteria was able to predict all type 1 ROP with 100-percent sensitivity and was able to reduce the number of typical ROP screening exams by roughly 30 percent. The authors admit that G-ROP screening criteria would need to be formally adopted by the guideline-governing bodies before most clinicians felt comfortable using it from a medical and legal standpoint. However, it’s easy to see the potential for more finely tuned ROP screening guidelines in the near future.
In addition to screening modifications, retinal imaging has been used to try to reduce the burden of ROP eye exams. The viability for remote ROP screening via carefully designed retinal imaging telehealth programs has been supported by many clinical studies and published reports from existing programs across the United States.7,8 At this time, telehealth ROP screening is endorsed, albeit with caution, by the AAP, AAPOS and AAO. The caveat being the need to follow the standard guidelines for optimal patient care with additional protocols to allow time for image reading, communication, bedside examination, transportation for care and other logistical issues that may arise.
The increased use of retinal imaging in ROP has spawned investigations into computer-based image analysis and artificial intelligence to facilitate ROP evaluation and management.8 A 2018 publication demonstrated that a fully automated algorithm trained with deep learning on retinal photographs was accurate, if not better, than its human counterparts at diagnosing plus disease.9 A meta-analysis of publications analyzing deep learning for ROP similarly demonstrated the systems’ high sensitivity and reliability in the detection and grading ROP in general.10 While this technology has yet to go mainstream, it represents an additional way in which retinal imaging has the potential to facilitate ROP care.
Lastly, while the acute phase of ROP is the focus of this discussion, we would be remiss if we didn’t recognize the increasing impact of long-term complications on our adult population of previously premature infants.
The latest ICROP guidelines highlight the various long-term sequelae that can affect infants with ROP as they grow into adulthood. In particular, the role that PAR plays in the development of reactivation of degenerative retinal disease in adulthood is getting more recognition. In the era of anti-VEGF treatments, the rate of PAR has been reported to be as high as 71 percent in treated infants.11 In addition, PAR has been associated with retinal holes and retinal detachments in late childhood and adulthood. All of these findings emphasize that a diagnosis of ROP in infancy signifies a risk for lifelong ocular anomalies. As our management of ROP evolves, so will its long-term effects on our adult population.
In conclusion, ICROP advises greater detail in description of ROP including:
- notch description to focal posterior disease;
- plus disease spectrum derived from zone I vessel appearance;
- re-classification of AP-ROP to A-ROP for disease inclusivity;
- re-classification of Stage 5 disease to forms -A, -B, and -C;
- level of disease regression;
- reactivation disease; and
- long-term sequelae.
Current screening guidelines from AAP/AAPOS still hold at this time but may be revised soon. Also, the number of infants screened may be able to be reduced with fine-tuned algorithms, and collaboration with the guideline governing bodies is paramount.
Telemedicine for ROP can be an appropriate care option for infants with limited access to in-person exams, and careful algorithms should be used for timely diagnosis and treatment. In addition, retinal image analysis has the potential to guide screening and therapeutic interventions. Clinicians should note that ROP portends a risk for lifelong ocular disease and patients/families should be counseled about this.
Dr. Collinge is an assistant professor in the Department of Pediatrics of the University of Connecticut School of Medicine. She can be reached at jcollinge@connecticutchildrens.org. She has no financial interest in any of the products discussed in the article.
1. The Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch Ophthalmol 1984;102:8:1130-4.
2. International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol 2005;123:7:991-9.
3. Chiang MF, Quinn GE, Fielder AR, et al. International classification of retinopathy of prematurity, third edition. Ophthalmology 2021;128:10:e51-e68.
4. Fierson WM, American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus, American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2018;142:6:e20183061.
5. Barrero-Castillero A, Corwin BK, VanderVeen DK, Wang JC. Workforce shortage for retinopathy of prematurity care and emerging role of telehealth and artificial intelligence. Pediatr Clin North Am 2020;67:4:725-733.
6. Binenbaum G, Tomlinson LA, de Alba Campomanes AG, et al. Validation of the postnatal growth and retinopathy of prematurity screening criteria. JAMA Ophthalmol 2020;138:1:31–37.
7. Quinn GE, Vinekar A. The role of retinal photography and telemedicine in ROP screening. Semin Perinatol 2019;43:6:367-374.
8. Valikodath N, Cole E, Chiang MF, et al. Imaging in retinopathy of prematurity. Asia Pac J Ophthalmol (Phila) 2019;8:2:178-186.
9. Brown JM, Campbell JP, Beers A, et al. Automated diagnosis of plus disease in retinopathy of prematurity using deep convolutional neural networks. JAMA Ophthalmol 2018;136:7:803-810.
10. Zhang J, Liu Y, Mitsuhashi T, Matsuo T. Accuracy of deep learning algorithms for the diagnosis of retinopathy of prematurity by fundus images: A systematic review and meta-analysis. J Ophthalmol 2021;2021:8883946.
11. Chang E, Rao P. Adult retinopathy of prematurity: Treatment implications, long term sequelae, and management. Curr Opin Ophthalmol 2921;32:5:489-493.



